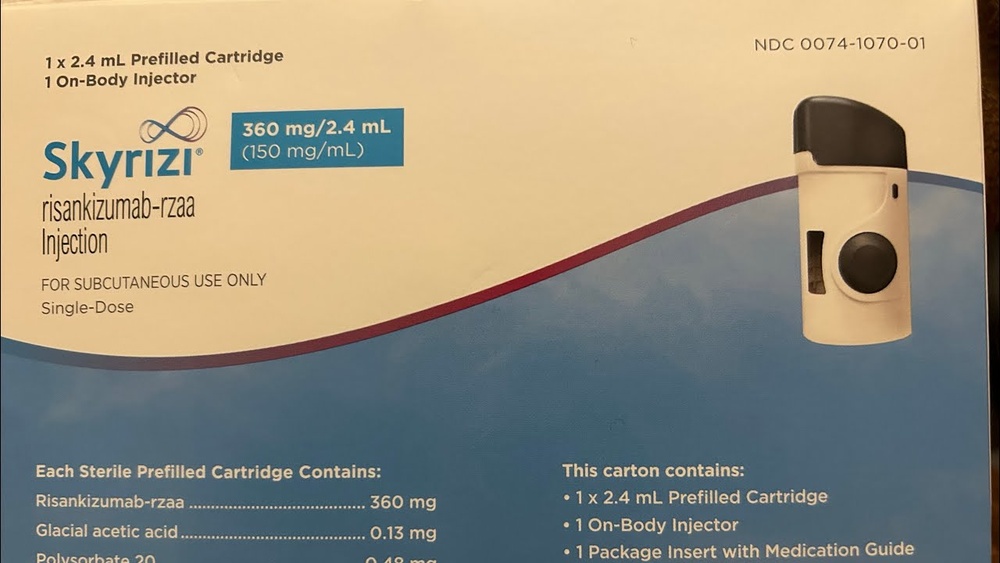
Инструкция по применению СКАЙРИЗИ 180 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В КАРТРИДЖЕ
Введение
Инструкция: информация для пациента
Skyrizi 180мг раствор для инъекций в картридже
Skyrizi 360мг раствор для инъекций в картридже
рисанкизумаб
Прочитайте внимательно всю инструкцию перед началом использования этого лекарства, поскольку она содержит важную информацию для вас.
- Сохраните эту инструкцию, поскольку вам может потребоваться прочитать ее снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой.
- Это лекарство было назначено только вам, и не передавайте его другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
Содержание инструкции
- Что такое Skyrizi и для чего оно используется
- Что вам нужно знать перед началом использования Skyrizi
- Как использовать Skyrizi
- Возможные побочные эффекты
- Хранение Skyrizi
- Содержание упаковки и дополнительная информация
- Инструкции по применению
1. Что такое Skyrizi и для чего оно используется
Skyrizi содержит активное вещество рисанкизумаб.
Skyrizi используется для лечения взрослых пациентов с:
- болезнью Крона средней и тяжелой степени
- язвенной колитой средней и тяжелой степени
Как работает Skyrizi
Это лекарство действует путем блокирования белка в организме, называемого "IL-23", который вызывает воспаление.
Болезнь Крона
Болезнь Крона - это воспалительное заболевание желудочно-кишечного тракта. Если у вас активная болезнь Крона, вам могут сначала назначить другие лекарства. Если эти лекарства не работают достаточно хорошо, вам будет назначен Skyrizi для лечения болезни Крона.
Язвенная колит
Язвенная колит - это воспалительное заболевание толстой кишки. Если у вас активная язвенная колит, вам могут сначала назначить другие лекарства. Если эти лекарства не работают достаточно хорошо или если вы не можете их принимать, вам будет назначен Skyrizi для лечения язвенной колита.
Skyrizi уменьшает воспаление и, таким образом, может помочь уменьшить симптомы вашего заболевания.
2. Что вам нужно знать перед началом использования Skyrizi
Не используйте Skyrizi
- если вы аллергичны к рисанкизумабу или к любому другому компоненту этого лекарства (указанному в разделе 6).
- если у вас есть инфекция, которую ваш врач считает значительной, например, активный туберкулез.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом, фармацевтом или медсестрой перед началом использования Skyrizi и во время лечения:
- если у вас есть текущая инфекция или если у вас есть инфекция, которая повторяется.
- если у вас есть туберкулез (ТБ).
- если вы недавно получили или планируете получить вакцину. Некоторые вакцины не должны быть введены во время лечения Skyrizi.
Важно сохранить копию номера партии Skyrizi.
Каждый раз, когда вы получаете новый упаковку Skyrizi, запишите дату и номер партии (указанный на упаковке после "Lot") и сохраните эту информацию в безопасном месте.
Аллергические реакции
Проконсультируйтесь с вашим врачом или обратитесь за медицинской помощью немедленно, если вы заметите какие-либо признаки аллергической реакции во время приема Skyrizi, например:
- затруднение дыхания или глотания
- отек лица, губ, языка или горла
- сильный зуд на коже, с красной сыпью или бугорками
Дети и подростки
Skyrizi не рекомендуется для детей и подростков моложе 18 лет, поскольку не было изучено использование Skyrizi в этой возрастной группе.
Другие лекарства и Skyrizi
Сообщите вашему врачу, фармацевту или медсестре:
- если вы используете, недавно использовали или можете использовать любое другое лекарство.
- если вы недавно получили вакцину или планируете получить вакцину. Некоторые вакцины не должны быть введены во время лечения Skyrizi.
В случае сомнений проконсультируйтесь с вашим врачом, фармацевтом или медсестрой перед использованием Skyrizi и во время лечения.
Беременность, контрацепция и лактация
Если вы беременны, думаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом перед использованием этого лекарства. Это необходимо, поскольку не известно, как это лекарство повлияет на ребенка.
Если вы женщина, способная стать беременной, вы должны использовать контрацепцию во время лечения этим лекарством и в течение как минимум 21 недели после последней дозы Skyrizi.
Если вы кормите грудью или планируете кормить грудью, проконсультируйтесь с вашим врачом перед использованием этого лекарства.
Вождение и использование машин
Маловероятно, что Skyrizi повлияет на вашу способность управлять транспортными средствами и использовать машины.
Skyrizi содержит натрий
Это лекарство содержит менее 1 ммоль натрия (23 мг) на картридж; это означает, что оно практически "не содержит натрия".
3. Как использовать Skyrizi
Следуйте точно инструкциям по введению этого лекарства, указанным вашим врачом или фармацевтом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом снова.
Это лекарство вводится путем инъекции под кожу (называемой "подкожной инъекцией").
Какое количество Skyrizi использовать
Вы начнете лечение Skyrizi с начальной дозы, которую введет ваш врач или медсестра путем внутривенного введения в руку (внутривенная инфузия).
Начальные дозы
Сколько? | Когда? | |
Болезнь Крона | 600 мг | Когда ваш врач укажет |
600 мг | Через 4 недели после 1-й дозы | |
600 мг | Через 4 недели после 2-й дозы |
Язвенная колит | Сколько? | Когда? |
1200 мг | Когда ваш врач укажет | |
1200 мг | Через 4 недели после 1-й дозы | |
1200 мг | Через 4 недели после 2-й дозы |
После этого вы будете получать Skyrizi путем инъекции под кожу.
Дозы поддержания
Болезнь Крона | Сколько? | Когда? |
1-я доза поддержания | 360 мг | Через 4 недели после последней начальной дозы (в 12-й неделе) |
Последующие дозы | 360 мг | Каждые 8 недель, начиная после 1-й дозы поддержания |
Язвенная колит | Сколько? | Когда? |
1-я доза поддержания | 180 мг или 360 мг | Через 4 недели после последней начальной дозы (в 12-й неделе) |
Последующие дозы | 180 мг или 360 мг | Каждые 8 недель, начиная после 1-й дозы поддержания |
Вы и ваш врач, фармацевт или медсестра решите, можно ли вам вводить это лекарство самостоятельно. Не вводите это лекарство самостоятельно, если ваш врач, фармацевт или медсестра не научили вас, как это делать. Также возможно, что это лекарство будет вводить человек, ухаживающий за вами, который научился делать это.
Прочитайте раздел7 "Инструкции по применению" в конце этой инструкции перед введением инъекции Skyrizi.
Если вы использовали больше Skyrizi, чем должно быть
Если вы использовали больше Skyrizi, чем должно быть, или ввели дозу раньше, чем было назначено, проконсультируйтесь с вашим врачом.
Если вы забыли использовать Skyrizi
Если вы забыли ввести Skyrizi, введите дозу как можно скорее. В случае сомнений проконсультируйтесь с вашим врачом.
Если вы прекратите лечение Skyrizi
Не прекращайте использование Skyrizi без предварительной консультации с вашим врачом. Если вы прекратите лечение, ваши симптомы могут вернуться.
Если у вас есть какие-либо другие вопросы о использовании этого лекарства, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Серьезные побочные эффекты
Проконсультируйтесь с вашим врачом или обратитесь за медицинской помощью немедленно, если у вас есть какие-либо симптомы серьезной инфекции, например:
- лихорадка, симптомы, похожие на грипп, ночные поты
- чувство усталости или трудности с дыханием, постоянный кашель
- жар, покраснение и боль на коже или болезненная сыпь с пузырьками
Ваш врач решит, можете ли вы продолжать использовать Skyrizi.
Другие побочные эффекты
Сообщите вашему врачу, фармацевту или медсестре, если вы заметите какие-либо из следующих побочных эффектов
Очень часто:могут возникать у более 1 из 10 человек
- инфекции верхних дыхательных путей с симптомами, такими как боль в горле и заложенность носа.
Часто:могут возникать у до 1 из 10 человек
- чувство усталости
- грибковые инфекции кожи
- реакции в месте инъекции (такие как покраснение или боль)
- зуд
- головная боль
- сыпь
- экзема
Редко:могут возникать у до 1 из 100 человек
- маленькие красные бугорки на коже
- крапивница
Сообщение о побочных эффектах
Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это возможные побочные эффекты, которые не указаны в этой инструкции. Также вы можете сообщить о них напрямую через систему мониторинга безопасности лекарственных средств: www.notificaRAM.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение Skyrizi
Храните это лекарство вне поля зрения и досягаемости детей.
Не используйте это лекарство после даты истечения срока годности, указанной на этикетке картриджа и на внешней упаковке после EXP.
Храните в холодильнике (при температуре между 2 °C и 8 °C). Не замораживайте.
Если необходимо, вы также можете хранить картридж вне холодильника (при максимальной температуре 25 °C) в течение максимум 24 часов.
Храните картридж в оригинальной упаковке, чтобы защитить его от света.
Не используйте это лекарство, если жидкость мутная или содержит чешуйки или крупные частицы.
Каждый инъектор для тела с картриджем предназначен для одноразового использования.
Лекарства не должны выбрасываться в канализацию или в мусор. Спросите вашего фармацевта, как утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Skyrizi
Активное вещество - рисанкизумаб.
Skyrizi 180 мг раствор для инъекций в картридже
- Каждый картридж содержит 180 мг рисанкизумаба в 1,2 мл раствора.
- Другие компоненты - тригидрат ацетата натрия, уксусная кислота, дигидрат трегалозы, полисорбат 20 и вода для инъекций.
Skyrizi 360 мг раствор для инъекций в картридже
- Каждый картридж содержит 360 мг рисанкизумаба в 2,4 мл раствора.
- Другие компоненты - тригидрат ацетата натрия, уксусная кислота, дигидрат трегалозы, полисорбат 20 и вода для инъекций.
Внешний вид продукта и содержание упаковки
Skyrizi - прозрачная бесцветная или желтоватая жидкость, содержащаяся в картридже. Жидкость может содержать крошечные прозрачные или белые частицы.
Каждый контейнер содержит 1 картридж и 1 инъекционный прибор для тела.
Владелец разрешения на продажу и ответственный за производство
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Людвигсхафен
Германия
Вы можете запросить дополнительную информацию о этом лекарстве, обратившись к местному представителю владельца разрешения на продажу:
Бельгия/Белгique/Бельгия AbbVie SA Тел.: +32 10 477811 | Литва AbbVie UAB Тел.: +370 5 205 3023 |
| Люксембург/Люксбург AbbVie SA Бельгия/Бельгия Тел.: +32 10 477811 |
Чешская Республика AbbVie s.r.o. Тел.: +420 233 098 111 | Венгрия AbbVie Kft. Тел.: +36 1 455 8600 |
Дания AbbVie A/S Тел.: +45 72 30-20-28 | Мальта V.J.Salomone Pharma Limited Тел.: +356 22983201 |
Германия AbbVie Deutschland GmbH & Co. KG Тел.: 00800 222843 33 (бесплатно) Тел.: +49 (0) 611 / 1720-0 | Нидерланды AbbVie B.V. Тел.: +31 (0)88 322 2843 |
Эстония AbbVie OÜ Тел.: +372 623 1011 | Норвегия AbbVie AS Тел.: +47 67 81 80 00 |
Греция AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Тел.: +30 214 4165 555 | Австрия AbbVie GmbH Тел.: +43 1 20589-0 |
Испания AbbVie Spain, S.L.U. Тел.: +34 91 384 09 10 | Польша AbbVie Sp. z o.o. Тел.: +48 22 372 78 00 |
Франция AbbVie Тел.: +33 (0) 1 45 60 13 00 | Португалия AbbVie, Lda. Тел.: +351 (0)21 1908400 |
Хорватия AbbVie d.o.o. Тел.: +385 (0)1 5625 501 | Румыния AbbVie S.R.L. Тел.: +40 21 529 30 35 |
Ирландия AbbVie Limited Тел.: +353 (0)1 4287900 | Словения AbbVie Biofarmacevtska družba d.o.o. Тел.: +386 (1)32 08 060 |
Исландия Vistor hf. Тел.: +354 535 7000 | Словакия AbbVie s.r.o. Тел.: +421 2 5050 0777 |
Италия AbbVie S.r.l. Тел.: +39 06 928921 | Финляндия AbbVie Oy Тел.: +358 (0)10 2411 200 |
Кипр Lifepharma (Z.A.M.) Ltd Тел.: +357 22 34 74 40 | Швеция AbbVie AB Тел.: +46 (0)8 684 44 600 |
Латвия AbbVie SIA Тел.: +371 67605000 |
Дата последнего обзора этого листка:
Другие источники информации
Подробная информация о этом лекарстве доступна на сайте Европейского агентства по лекарственным средствам: http://www.ema.europa.eu.
Подробная и актуальная информация о этом продукте доступна ниже или на внешней упаковке, сканируя QR-код через смартфон. Та же информация также доступна на сайте: www.skyrizi.eu
QR-код для включения
Чтобы запросить копию этого листка в
- Инструкции по применению
Прочитайте весь раздел7 перед использованием Skyrizi
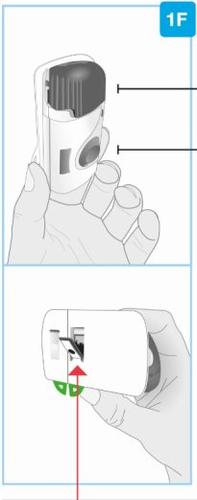
Инъекционный прибор Skyrizi
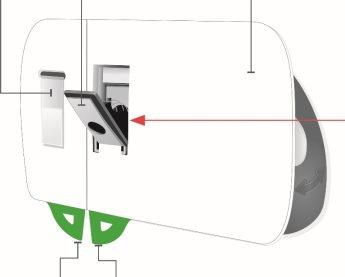
Фронтальный вид Материал адгезивный | Свет индикатора состояния | Кнопка начала Не трогайте ее до тех пор, пока не будете готовы сделать инъекцию |
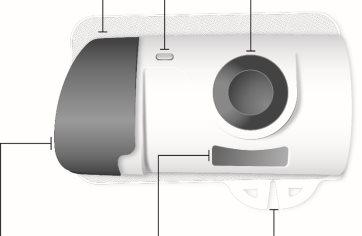
Серая дверца Не закрывайте серую дверцу без картриджа внутри | Окно лекарства | Петли |
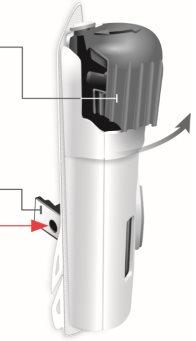
Задний вид
Прозрачная пластиковая полоска | Защитник иглы | Адгезивное покрытие |
| Будьте осторожны. Игла внутри (под защитником иглы) Не трогайте зону защитника иглы или иглу | |
Зеленая петля небольшого участка | Зеленая петля большого участка |
Боковой вид
Закрытие дверцы Боковая часть дверцы имеет пазы Серая дверца должна быть слегка приоткрыта Не закрывайте серую дверцу без картриджа внутри Защитник иглы Игла внутри (под защитником иглы) Не трогайте зону защитника иглы или иглу |
|
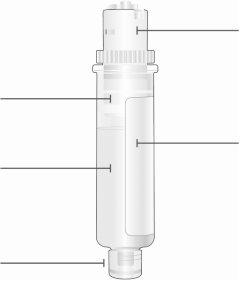
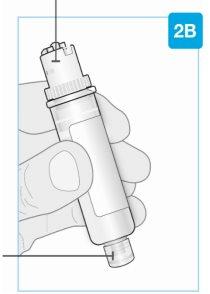
Картридж
Белый поршень перемещается по камере к нижней части картриджа при инъекции лекарства. Лекарство Нижняя часть меньшего размера |
| Верхняя часть большего размера картриджа Не поворачивайте и не удаляйте Срок годности (EXP) Находится на этикетке картриджа |
Важная информация, которую необходимо знать перед инъекцией Skyrizi
- Вы должны пройти обучение по инъекции Skyrizi перед тем, как сделать инъекцию. Если вам нужна помощь, обратитесь к вашему врачу, фармацевту или медсестре
- Отметьте даты в календаре, чтобы знать, когда вам необходимо сделать инъекцию Skyrizi
- Инъекционный прибор для тела одноразового использования предназначен только для использования с картриджем Skyrizi
- Храните Skyrizi в оригинальной упаковке, чтобы защитить лекарство от света до момента использования
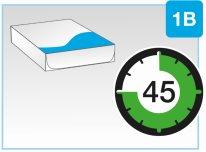
- Удалите упаковку из холодильника и оставьте ее при комнатной температуре, подальше от прямого солнечного света, не менее 45 и до 90минутперед инъекцией
- Непозволяйте инъекционному прибору для тела намокнуть водой или любым другим жидкостью
- Нетрогайте кнопку начала до тех пор, пока не поместите инъекционный прибор для тела, загруженный с картриджем, на кожу и не будете готовы сделать инъекцию
- Вы можете нажать кнопку начала только одинраз
- Во время процесса инъекции необходимо ограничить физическую активность. Можно выполнять умеренные физические упражнения, такие как ходьба, растяжка или сгибание
- Неоткладывайте инъекцию лекарства после загрузки чистого картриджа в инъекционный прибор для тела. Если вы подождете, лекарство высохнет, и инъекционный прибор для тела перестанет работать
- Неделайте инъекцию лекарства, если жидкость в окне осмотра мутная или содержит чешуйки или крупные частицы. Жидкость должна быть прозрачной или желтого цвета и может содержать крошечные прозрачные или белые частицы
- Нетрясите упаковку, картридж или инъекционный прибор для тела
- Неповторно используйте картридж или инъекционный прибор для тела
Верните это лекарство в аптеку
- после даты истечения срока годности (EXP), указанной на упаковке
- если жидкость была заморожена в любое время (даже если она была разморожена)
- если картридж или инъекционный прибор для тела были уронены или повреждены
- если отверстия упаковки повреждены
- если белая бумажная обертка упаковки отсутствует или повреждена
Следуйте следующим шагам каждый раз, когда используете Skyrizi
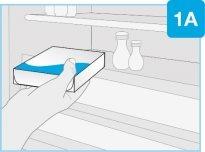
ШАГ1: Подготовьтесь | |
| Удалите упаковку из холодильника и оставьте ее при комнатной температуре, подальше от прямого солнечного света, не менее 45 и до 90минутперед инъекцией.
|
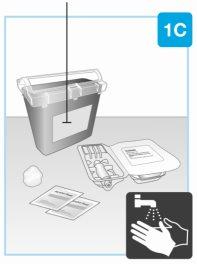
Контейнер для специальных отходов
| Соберите все материалы и вымойте руки На гладкой и чистой поверхности положите следующее:
Вымойте и высушите руки. |
| Удалите бумажную обертку с поддона
Поднимите пластиковую крышку
|
Серая дверца Кнопка начала Игла внутри(под защитником иглы) | Осмотрите инъекционный прибор для тела
Если вы нажмете серую кнопку начала до того, как поместите инъекционный прибор для тела на тело, вы не сможете использовать инъекционный прибор для тела. Если это произойдет, обратитесь к вашему врачу, фармацевту или медсестре. |
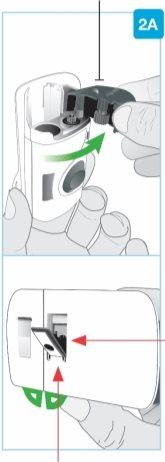
ШАГ2: Подготовка инъекционного прибора для тела | |
Серая дверца
Задний вид Игла внутри(под защитником иглы) Защитник иглы | Откройте серую дверцу полностью
Положите инъекционный прибор для тела в сторону. |
Верхняя часть большего размера картриджа
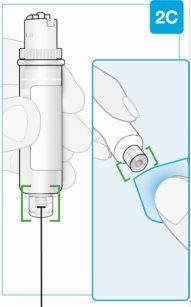
Нижняя часть меньшего размера | Осмотрите картридж Аккуратно удалите картридж из пластиковой поддона.
Проверьте картридж
|
Нижняя часть меньшего размера Очистите центр нижней части меньшего размера | Очистите нижнюю часть меньшего размера картриджа Найдите нижнюю часть меньшего размера картриджа
|
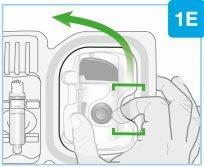
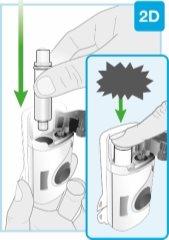
Вставьте прямо “щелчок”
| Загрузите чистый картридж в инъекционный прибор для тела
Убедитесь, что вы переходите к следующему шагу немедленно. Если вы подождете, лекарство высохнет. |
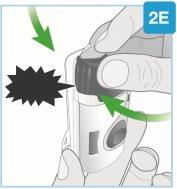
“щелчок” | Закройте серую дверцу Поверните серую дверцу влево, затем нажмите крепко и услышите “щелчок” при закрытии серой дверцы
|
ШАГ3: Подготовка к инъекции | |
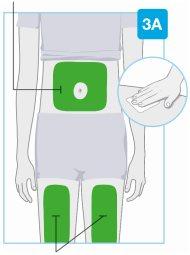
Зоны инъекции
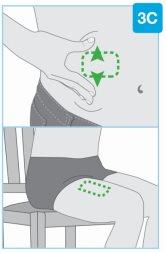
Зоны инъекции | Выберите и очистите место инъекции Выберите одну из этих 3 зон для инъекции:
Неделайте инъекцию в зонах кожи с складками или естественными выступами, поскольку инъекционный прибор для тела может упасть во время использования. Перед инъекцией очистите место инъекции салфеткой, пропитанной спиртом, делая круговые движения.
|
Небольшой участок Большой участок
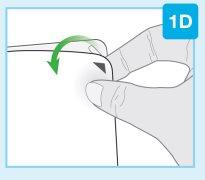
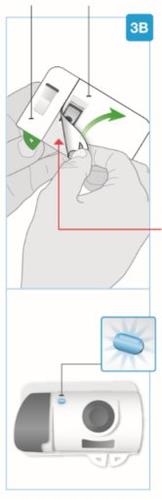
Игла внутри (под защитником иглы) Активированный инъектор Свет индикатора мигает синим | Отделите обе петли, чтобы открыть адгезивную кожу Переверните инъекционный прибор для тела, чтобы найти две зеленые петли
Удалите большую часть, используя зеленую петлю, чтобы открыть адгезивную кожу Удалите небольшую часть, используя зеленую петлю, чтобы открыть адгезивную кожу. Таким образом, вы удалите прозрачную пластиковую полоску и активируете инъекционный прибор для тела.
|
- Удалите защитный материал с корпуса инъектора, не допуская приклеивания клейкой части к себе
Корпоральный инъектор Skyrizi следует размещать на коже, а инъекцию необходимо начинать в течение 30 минут после удаления зеленых вкладышей; в противном случае он не будет работать. Убедитесь, что переходите к следующему шагу сразу же.

Если состояние индикатора мигает красным, корпоративный инъектор не работает правильно. Не продолжайте использовать его.
Обратитесь к вашему врачу, фармацевту или медсестре за помощью.
Если корпоративный инъектор приклеен к вашему телу, осторожно удалите его с кожи.
Подготовьте корпоративный инъектор для установки
- Для живота необходимо переместить и удерживать кожу, чтобы создать твердую и ровную поверхность для инъекции, находящуюся на расстоянии не менее 5 см от пупка. Убедитесь, что вы сидите прямо, чтобы избежать складок и выступов кожи.
- Не нужно тянуть кожу, чтобы выровнять переднюю часть левого или правого бедра.
Убедитесь, что корпоративный инъектор размещен так, чтобы вы могли видеть синий индикатор.
Разместите корпоративный инъектор на коже
- Когда синий свет начнет мигать, инъектор будет готов. Поместите инъектор на чистую кожу с видимым индикатором
- Неразмещайте инъектор на одежде. Размещайте его только на голой коже.
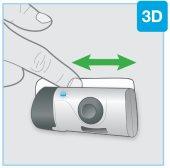
- Проведите пальцем вокруг клеевого материала, чтобы закрепить его
- Неперемещайте и не регулируйте инъектор после его размещения на коже
Перейдите к следующему шагу немедленно.
ШАГ4: Введение Skyrizi
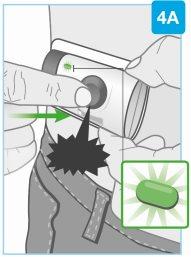
«щелчок»
Начните инъекцию
Твердamente нажмите серую кнопку запуска и отпустите ее
- Вы услышите «щелчок» и можете почувствовать укол иглы
- Проверьте индикатор, когда инъектор издаст сигнал
- После начала инъекции индикатор будет непрерывно мигать зеленым светом
- После начала инъекции вы услышите звуки насоса, пока инъектор вводит лекарство
Непродолжайте использовать инъектор, если индикатор мигает красным. Удалите его с кожи, если индикатор мигает красным. Если это происходит, сообщите об этом вашему врачу, фармацевту или медсестре.
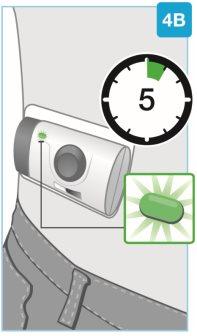
Подождите, пока инъекция не будет завершена
- Может потребоваться до 5 минут, чтобы полностью ввести лекарство. Корпоративный инъектор автоматически остановится, когда инъекция будет завершена
- Во время инъекции индикатор будет продолжать мигать зеленым светом
- Во время инъекции вы услышите звуки насоса, пока инъектор продолжает вводить лекарство
- Во время инъекции можно выполнять умеренные физические упражнения, такие как ходьба, растяжка или сгибание.
Непродолжайте использовать инъектор, если индикатор мигает красным. Удалите его с кожи, если индикатор мигает красным. Если это происходит, сообщите об этом вашему врачу, фармацевту или медсестре.
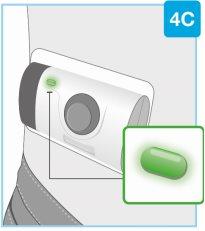
Инъекция завершена, когда:
- Корпоративный инъектор автоматически останавливается
- Звучит сигнал, и индикатор меняется на стабильный зеленый свет. Если индикатор изменился на стабильный зеленый, это означает, что инъекция завершена
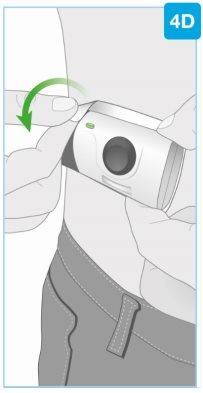
Удалите инъектор
- Непомещайте пальцы на заднюю часть инъектора при его удалении с кожи
- После завершения инъекции возьмите угол клеевого материала, чтобы осторожно удалить инъектор с кожи
- Избегайте прикосновения к защитному покрытию иглы или самой игле на задней части инъектора
- После удаления инъектора вы услышите несколько сигналов, и индикатор погаснет
- Защитное покрытие иглы закроет иглу, когда инъектор будет удален с кожи
- Нормально, если на коже после удаления инъектора появятся небольшие капли жидкости
- Нажмите ватный шарик или марлю на место инъекции и удерживайте в течение 10 секунд.
- Нетрите место инъекции
- Нормально, если на месте инъекции появится небольшое кровотечение
Перейдите к следующему шагу.
ШАГ5: Завершение
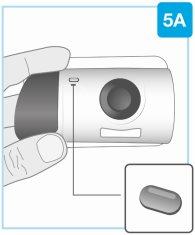
Проверьте инъектор
Осмотрите окно препарата и индикатор.
Убедитесь, что белый поршень заполняет все окно препарата, и что стабильный зеленый свет гаснет, что указывает на то, что все лекарство было введено.
- Если белый поршень не заполняет окно, сообщите об этом вашему врачу, фармацевту или медсестре
Контейнер для специальных отходов
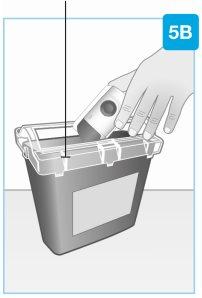
Утилизация
Утилизируйте использованный корпоративный инъектор в контейнере для специальных отходов сразу после использования.
- Корпоративный инъектор содержит батареи, электронные компоненты и иглу
- Оставьте картридж в инъекторе.
- Невыбрасывайте использованный инъектор в домашний мусор
- Ваш врач, фармацевт или медсестра объяснят, как вернуть контейнер для специальных отходов, когда он будет заполнен. Возможно, существуют местные рекомендации по утилизации
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги СКАЙРИЗИ 180 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В КАРТРИДЖЕФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 150 мгАктивное вещество: РисанкизумабПроизводитель: Abbvie Deutschland Gmbh & Co. KgТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 150 мгАктивное вещество: РисанкизумабПроизводитель: Abbvie Deutschland Gmbh & Co. KgТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 360 мгАктивное вещество: РисанкизумабПроизводитель: Abbvie Deutschland Gmbh & Co. KgТребуется рецепт
Аналоги СКАЙРИЗИ 180 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В КАРТРИДЖЕ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог СКАЙРИЗИ 180 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В КАРТРИДЖЕ в Ukraine
Врачи онлайн по СКАЙРИЗИ 180 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В КАРТРИДЖЕ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на СКАЙРИЗИ 180 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В КАРТРИДЖЕ – по решению врача и с учетом местных правил.