
SILVEDERMA 10 mg/g CREMA

Cómo usar SILVEDERMA 10 mg/g CREMA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Silvederma 10 mg/g crema
Sulfadiazina de plata
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento porque contiene información importante para usted.
|
Contenido del prospecto:
- Qué es Silvederma 10 mg/g crema y para qué se utiliza
- Qué necesita saber antes de usar Silvederma 10 mg/g crema
- Cómo usar Silvederma 10 mg/g crema
- Posibles efectos adversos
- Conservación de Silvederma 10 mg/g crema
- Contenido del envase e información adicional
1. Qué es Silvederma 10 mg/g crema y para qué se utiliza
Este medicamento contiene sulfadiazina de plata, que es un antibiótico del grupo de las sulfamidas (medicamento útil para el tratamiento de infecciones).
Los antibióticos se utilizan para tratar infecciones bacterianas y no sirven para tratar infecciones víricas.
Es importante que siga las instrucciones relativas a la dosis, el intervalo de administración y la duración del tratamiento indicadas por su médico.
No guarde ni reutilice este medicamento. Si una vez finalizado el tratamiento le sobra antibiótico, devuélvalo a la farmacia para su correcta eliminación. No debe tirar los medicamentos por el desagüe ni a la basura.
Este medicamento está indicado en el tratamiento y prevención de infecciones en quemaduras de segundo y tercer grado, así como en úlceras varicosas y de decúbito.
2. Qué necesita saber antes de usar Silvederma 10 mg/g crema
No use Silvederma 10 mg/g crema:
- Si es alérgico (hipersensible) a la sulfadiazina argéntica, las sulfamidas (grupo al que pertenece este medicamento), o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- En caso de lesiones de gran superficie en niños recién nacidos, prematuros, durante los últimos días del embarazo o durante la lactancia.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Silvederma 10 mg/g crema.
- Se han descrito erupciones cutáneas que pueden amenazar la vida del paciente (síndrome de Stevens Johnson y necrólisis epidérmica toxica) con el uso de Silvederma 10 mg/g crema, inicialmente aparecen como puntos o manchas circulares rojizos, a menudo con una ampolla central.
- Otros signos adicionales que pueden aparecer son llagas en la boca, garganta, nariz, genitales y conjuntivitis (ojos hinchados y rojos).
- Estas erupciones en la piel que pueden amenazar la vida del paciente, a menudo van acompañadas de síntomas de gripe. La erupción puede progresar a la formación de ampollas generalizadas o descamación de la piel.
- El periodo de mayor riesgo de aparición de reacciones cutáneas graves es durante las primeras semanas de tratamiento.
- Si usted ha desarrollado síndrome de Stevens Johnson o necrólisis Epidérmica Tóxica con el uso de Silvederma 10 mg/g crema, no debe utilizar Silvederma crema de nuevo en ningún momento.
- Si usted desarrolla erupciones o estos síntomas en la piel deje de usar Silvederma 10 mg/g crema, acuda inmediatamente a un médico e infórmele de que usted está utilizando este medicamento.
Tenga especial cuidado con Silvederma 10 mg/g crema:
- Si padece alguna enfermedad de hígado o riñón, debe evitar la aplicación de la crema en lesiones de gran superficie o abiertas, sobre todo úlceras.
- Si padece una reducción del número de glóbulos blancos en la sangre, su médico le realizará recuentos de control.
- Si padece un déficit del enzima glucosa-6-fosfato deshidrogenasa.
- No debe exponer la zona tratada con este medicamento a la luz directa del sol, ya que puede producirse una decoloración de la piel, así como una coloración gris de la crema.
Uso de Silvederma 10 mg/g crema con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No debe usar este medicamento durante los últimos días del embarazo o durante el periodo de lactancia.
Conducción y uso de máquinas
Es poco probable que este medicamento ejerza algún efecto sobre la capacidad para conducir vehículos o manejar maquinaria.
Silvederma contiene propilenglicol, alcohol cetoestearílico y parahidroxibenzoato de metilo (E218)
Este medicamento puede producir irritación de la piel porque contiene propilenglicol.
Este medicamento puede producir reacciones locales en la piel (como dermatitis de contacto) porque contiene alcohol cetoesterarílico.
Este medicamento puede producir reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de metilo (E-218).
3. Cómo usar Silvederma 10 mg/g crema
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmaceútico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Se administra de forma superficial por vía cutánea.
Inicialmente debe lavar y limpiar la herida. Después, con una espátula estéril o con la mano cubierta con un guante estéril, debe aplicar una capa de 3 mm de espesor sobre la superficie lesionada, cubriéndola con un vendaje adecuado.
Normalmente la renovación del vendaje se realizará 1-2 veces al día, pudiendo renovarse cada 4-6 horas en el caso de heridas muy contaminadas.
En cada cambio de vendaje y reposición del medicamento, se deben eliminar primero los restos de la aplicación anterior, lavando cuidadosamente la herida con agua hervida tibia o solución salina isotónica.
Cada envase debe ser utilizado para un solo paciente.
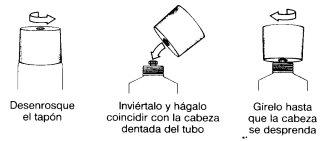
Instrucciones para la correcta administración del preparado:
El tubo tiene un precinto cuya apertura se realiza siguiendo las instrucciones del dibujo:
Si usa más Silvederma 10 mg/g crema del que debe
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida o utilizada.
Si olvidó usar Silvederma10 mg/g crema
Si olvida aplicar una dosis, aplíquesela en cuanto se acuerde. No tome una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efecto adversos muy raros (puede afectar hasta 1 de cada 10.000 personas):
- Pueden aparecer erupciones en la piel que pueden amenazar la vida del paciente (síndrome de Stevens Johnson, necrólisis epidérmica tóxica) (ver sección 2).
- Efectos adversos con una frecuencia no conocida (no puede estimarse a partir de los datos disponibles). Si observa alguna de las siguientes reacciones, informe inmediatamente a su médico:Reacciones alérgicas.
- Reacciones cutáneas como sensación de quemazón o dolor.
- Decoloración gris de la piel en la zona de aplicación por exposición solar.
- Reducción del número de glóbulos blancos en la sangre (leucopenia).
- Aumento de osmolalidad en suero.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Silvederma 10 mg/g crema
Mantener ete medicamento fuera de la vista y del alcance de los niños.
No requiere condiciones especiales de conservación. Conservar en el embalaje original para protegerlo de la luz.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Silvederma 10 mg/g crema:
- El principio activo es sulfadiazina de plata. Cada gramo de crema contiene 10 miligramos de sulfadiazina de plata.
- Los demás componentes son: Alcohol cetoestearílico, miristato de isopropilo, vaselina blanca, polioxil 40 estearato, oleato de sorbitan, propilenglicol (E-1520), parahidroxibenzoato de metilo (E-218) y agua purificada.
Aspecto del producto y contenido del envase
Silvederma10 mg/g crema es una crema de color blanco o blanco-hueso, que se presenta en tubos con 50 gramos ó 100 gramos y en tarro con 500 gramos de crema. Se acondiciona en caja de cartón.
Titular de la autorización de comercialización y responsable de la fabricación:
Laboratorio ALDO-UNIÓN, S.L.
Calle Baronesa de Maldá, 73
08950 Esplugues de Llobregat
BARCELONA – ESPAÑA
Este prospecto fue aprobado en mayo de 2007
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SILVEDERMA 10 mg/g CREMAForma farmacéutica: CREMA, 10 mg sulfadiazina argentica/gPrincipio activo: Sulfadiazina argenticaFabricante: Alliance Pharma (Ireland) LimitedRequiere recetaForma farmacéutica: LIQUIDO USO TOPICO, 10 mg/mlPrincipio activo: Sulfadiazina argenticaFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: CREMA, -Principio activo: silver sulfadiazine, combinationsFabricante: Alliance Pharma (Ireland) LimitedRequiere receta
Médicos online para SILVEDERMA 10 mg/g CREMA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SILVEDERMA 10 mg/g CREMA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








