OMVOH 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar OMVOH 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Omvoh 100 mg solución inyectable en pluma precargada
mirikizumab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Omvoh y para qué se utiliza
- Qué necesita saber antes de empezar a usar Omvoh
- Cómo usar Omvoh
- Posibles efectos adversos
- Conservación de Omvoh
- Contenido del envase e información adicional
1. Qué es Omvoh y para qué se utiliza
Omvoh contiene el principio activo mirikizumab, un anticuerpo monoclonal. Los anticuerpos monoclonales son proteínas que identifican y se unen específicamente a ciertas proteínas diana del organismo. Omvoh actúa uniéndose y bloqueando una proteína del organismo llamada IL-23 (interleucina-23), que está implicada en la inflamación. Al bloquear la acción de la IL-23, Omvoh reduce la inflamación y otros síntomas asociados con la colitis ulcerosa.
Colitis ulcerosa
La colitis ulcerosa es una enfermedad inflamatoria crónica del intestino grueso. Si padece colitis ulcerosa, le administrarán primero otros medicamentos. Si no responde suficientemente bien o no puede tolerar estos medicamentos, puede que le administren Omvoh para reducir los signos y síntomas de la colitis ulcerosa, como diarrea, dolor abdominal, urgencia y sangrado rectal.
2. Qué necesita saber antes de empezar a usar Omvoh
No use Omvoh
- si es alérgico a mirikizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Si cree que puede ser alérgico, consulte a su médico antes de utilizar Omvoh.
- si tiene infecciones activas importantes (tuberculosis activa).
Advertencias y precauciones
- Consulte a su médico o farmacéutico antes de empezar a usar este medicamento.
- Su médico comprobará cómo se encuentra antes del tratamiento.
- Asegúrese de informar a su médico sobre cualquier enfermedad que sufra antes del tratamiento.
Infecciones
- Omvoh puede potencialmente causar infecciones graves. No se debe iniciar el tratamiento con Omvoh si tiene una infección activa hasta que la infección desaparezca.
- Después de comenzar el tratamiento, informe a su médico de inmediato si tiene algún síntoma de infección, como:
|
|
|
|
|
|
|
|
- Informe también a su médico si ha estado recientemente cerca de alguien que pudiera tener tuberculosis.
- Su médico le examinará y le hará un test para la detección de la tuberculosis antes de usar Omvoh.
- Si su médico cree que usted está en riesgo de padecer tuberculosis activa, puede que le administre medicamentos para tratarla.
Vacunas
Su médico comprobará si necesita alguna vacuna antes de iniciar el tratamiento. Informe a su médico, farmacéutico o enfermero si se ha vacunado recientemente o se va a vacunar. Algunos tipos de vacunas (vacunas vivas) no deben administrarse mientras se usa Omvoh.
Reacciones alérgicas
- Omvoh puede causar potencialmente reacciones alérgicas graves.
- Deje de usar Omvoh y busque atención médica de inmediato si presenta cualquiera de los siguientes síntomas de una reacción alérgica grave:
|
|
|
|
|
|
Pruebas sanguíneas del hígado
Su médico le realizará un análisis de sangre antes de iniciar el tratamiento con Omvoh y durante el mismo para comprobar si su hígado funciona con normalidad. Si los análisis de sangre son anormales, su médico podría interrumpir el tratamiento con Omvoh y realizar pruebas adicionales en su hígado para determinar la causa.
Niños y adolescentes
No se recomienda el uso de Omvoh en niños y adolescentes menores de 18 años ya que no se ha estudiado en este grupo de edad.
Otros medicamentos y Omvoh
Informe a su médico, farmacéutico o enfermero
- si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
- si ha sido vacunado recientemente o va a vacunarse. No se deben administrar determinados tipos de vacunas (vacunas vivas) mientras se utilice Omvoh.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Es preferible evitar el uso de Omvoh en el embarazo. Se desconocen los efectos de Omvoh en mujeres embarazadas. Si es una mujer en edad fértil, se le recomienda que evite quedarse embarazada y que utilice un método anticonceptivo adecuado mientras esté utilizando Omvoh y durante al menos 10 semanas después de la última dosis de Omvoh.
Si está en periodo de lactancia, o tiene intención de estarlo, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Es poco probable que Omvoh influya en su capacidad para conducir y utilizar máquinas.
Omvoh contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
Omvoh contiene polisorbato
Este medicamento contiene 0,3 mg/ml de polisorbato 80 en cada pluma equivalente a 0,6 mg para la dosis de mantenimiento para tratar la colitis ulcerosa. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene cualquier alergia conocida.
3. Cómo usar Omvoh
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o enfermero. En caso de duda, consulte de nuevo a su médico, enfermero o farmacéutico.
Qué cantidad de Omvoh se administra y durante cuánto tiempo
Su médico decidirá la cantidad de Omvoh que necesita y la duración del tratamiento. Omvoh es para tratamiento a largo plazo. Su médico o enfermero monitorizará periódicamente su estado para comprobar que el tratamiento está teniendo el efecto deseado.
Colitis ulcerosa
- Inicio del tratamiento: la primera dosis de Omvoh es de 300 mg y su médico se la administrará mediante perfusión intravenosa (goteo en una vena del brazo) durante al menos 30 minutos.
Después de la primera dosis, recibirá otra dosis de 300 mg de Omvoh 4 semanas después y de nuevo, al cabo de otras 4 semanas.
Si no tiene una respuesta terapéutica adecuada después de estas 3 perfusiones, su médico podría considerar continuar con las perfusiones intravenosas en las semanas 12, 16 y 20.
- Tratamiento de mantenimiento: 4 semanas después de la última perfusión intravenosa, se administrará una dosis de mantenimiento de 200 mg de Omvoh mediante inyección bajo la piel (“por vía subcutánea”) y después, cada 4 semanas. La dosis de mantenimiento de 200 mg se administrará mediante 2 inyecciones de 100 mg de Omvoh cada una.
Si pierde la respuesta después de recibir la dosis de mantenimiento de Omvoh, su médico puede decidir administrarle 3 dosis de Omvoh mediante perfusiones intravenosas.
Su médico o enfermero le indicarán cuándo cambiar a las inyecciones subcutáneas.
Durante el tratamiento de mantenimiento, usted y su médico o enfermero deben decidir si debe inyectarse Omvoh usted mismo después de recibir formación en la técnica de inyección subcutánea. Es importante que no intente inyectarse usted mismo hasta que su médico o enfermero le hayan enseñado. Su médico o enfermero le proporcionarán la formación necesaria. Un cuidador también puede administrarle la inyección de Omvoh después de la formación adecuada.
Use un método de recordatorio, como notas en un calendario o diario, para ayudarle a recordar cuando administrar su próxima dosis para evitar omitir o repetir la dosis.
Si recibe más Omvoh del que debe
Si ha recibido más Omvoh del que debe o la dosis se ha administrado antes de lo prescrito, informe a su médico.
Si olvidó usar Omvoh
Si ha olvidado inyectarse una dosis de Omvoh, inyéctesela lo antes posible. A partir de entonces, retome la administración cada 4 semanas.
Si interrumpe el tratamiento con Omvoh
No debe interrumpir el tratamiento con Omvoh sin consultar antes con su médico. Si interrumpe el tratamiento, los síntomas de la colitis ulcerosa pueden volver a aparecer.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Reacciones en el lugar de inyección (p.ej.: enrojecimiento de la piel, dolor)
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Infecciones del tracto respiratorio superior (infecciones de nariz y garganta)
- Dolor de las articulaciones
- Dolor de cabeza
- Erupción cutánea
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Herpes
- Reacción alérgica relacionada con la perfusión (p.ej.: picor, urticaria)
- Aumento del nivel de enzimas hepáticas en sangre.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Omvoh
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en el embalaje exterior después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar.
Nocaliente las plumas en el microondas, no las moje con agua caliente, ni las exponga a la luz solar directa.
Noagite la pluma precargada.
Conservar en el embalaje original para protegerlo de la luz.
Omvoh puede almacenarse sin refrigerar hasta 2 semanas a una temperatura no superior a 30 ºC.
Si se superan estas condiciones, se debe desechar Omvoh.
No utilice este medicamento si observa que la pluma precargada está dañada, o el medicamento está turbio, considerablemente marrón, o tiene partículas.
Este medicamento es para un solo uso.
Los medicamentos no se deben tirar por los desagües. Pregunte a su médico, enfermero o farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Omvoh
- El principio activo es mirikizumab.
Cada pluma precargada contiene 100 mg de mirikizumab en 1 ml de solución.
- Los demás componentes son histidina; monoclorhidrato de histidina; cloruro de sodio; manitol (E 421); polisorbato 80 (E 433); agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Omvoh es una solución en un cartucho de vidrio transparente insertada en una pluma desechable, para un solo uso. Su color puede variar de incoloro a ligeramente amarillo.
Omvoh se presenta en envases que contienen 2 plumas precargadas, en envases múltiples de 2 cartonajes, que contienen cada uno 2 plumas precargadas y en envases múltiples de 3 cartonajes, que contienen cada uno 2 plumas precargadas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Eli Lilly Nederland B.V.,
Papendorpseweg 83
3528 BJ Utrecht
Países Bajos
Responsable de la fabricación
Lilly France S.A.S.
Rue du Colonel Lilly
67640 Fegersheim
Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Belgique/België/Belgien Eli Lilly Benelux S.A./N.V. Tél/Tel: + 32-(0)2 548 84 84 | Lietuva Eli Lilly Lietuva Tel. +370 (5) 2649600 |
| Luxembourg/Luxemburg Eli Lilly Benelux S.A./N.V. Tél/Tel: + 32-(0)2 548 84 84 |
Ceská republika ELI LILLY CR, s.r.o. Tel: + 420 234 664 111 | Magyarország Lilly Hungária Kft. Tel: + 36 1 328 5100 |
Danmark Eli Lilly Danmark A/S Tlf.: +45 45 26 60 00 | Malta Charles de Giorgio Ltd. Tel: + 356 25600 500 |
Deutschland Lilly Deutschland GmbH Tel. + 49-(0) 6172 273 2222 | Nederland Eli Lilly Nederland B.V. Tel: + 31-(0) 30 60 25 800 |
Eesti Eli Lilly Nederland B.V. Tel: +372 6 817 280 | Norge Eli Lilly Norge A.S. Tlf: + 47 22 88 18 00 |
Ελλáδα ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε. Τηλ: +30 210 629 4600 | Österreich Eli Lilly Ges.m.b.H. Tel: + 43-(0) 1 711 780 |
España Lilly S.A. Tel: + 34-91 663 50 00 | Polska Eli Lilly Polska Sp. z o.o. Tel: +48 22 440 33 00 |
France Lilly France Tél: +33-(0) 1 55 49 34 34 | Portugal Lilly Portugal Produtos Farmacêuticos, Lda Tel: + 351-21-4126600 |
Hrvatska Eli Lilly Hrvatska d.o.o. Tel: +385 1 2350 999 | România Eli Lilly România S.R.L. Tel: + 40 21 4023000 |
Ireland Eli Lilly and Company (Ireland) Limited Tel: + 353-(0) 1 661 4377 | Slovenija Eli Lilly farmacevtska družba, d.o.o. Tel: +386 (0)1 580 00 10 |
Ísland Icepharma hf. Sími + 354 540 8000 | Slovenská republika Eli Lilly Slovakia, s.r.o. Tel: + 421 220 663 111 |
Italia Eli Lilly Italia S.p.A. Tel: + 39- 055 42571 | Suomi/Finland Oy Eli Lilly Finland Ab Puh/Tel: + 358-(0) 9 85 45 250 |
Κúπρος Phadisco Ltd Τηλ: +357 22 715000 | Sverige Eli Lilly Sweden AB Tel: + 46-(0) 8 7378800 |
Latvija Eli Lilly (Suisse) S.A. Parstavnieciba Latvija Tel: +371 67364000 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu, y en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (https://www.aemps.gob.es/).
Instrucciones de uso Omvoh 100mg solución inyectable en pluma precargada mirikizumab 2plumas precargadas: 1 pluma de 100mg y 1 pluma de 100mg
|
Lea esto antes de inyectar Omvoh. Siga todas las instrucciones paso a paso.
|
También tenga en cuenta:
- Su profesional sanitario debe enseñarle cómo preparar e inyectar Omvoh utilizando la pluma. Nose inyecte usted mismo o inyecte a otra persona hasta que le hayan mostrado cómo inyectar Omvoh.
- Cada pluma de Omvoh es para un solo uso. No comparta o reutilice su pluma. Puede transmitir o que le transmitan una infección.
- Su profesional sanitario puede ayudarle a decidir en qué zona de su cuerpo inyectar su dosis. También puede leer en estas instrucciones la sección “Elija su lugar de inyección” para ayudarle a escoger qué zona puede ser mejor para usted.
- Si tiene problemas de visión o audición, no use la pluma de Omvoh sin la ayuda de un cuidador.
- Guarde las instrucciones de uso y vuelva a leerlas si lo necesita.
Antes de usar las plumas de Omvoh, lea y siga de forma cuidadosa todas las instrucciones paso a paso.
Partes de la pluma de Omvoh
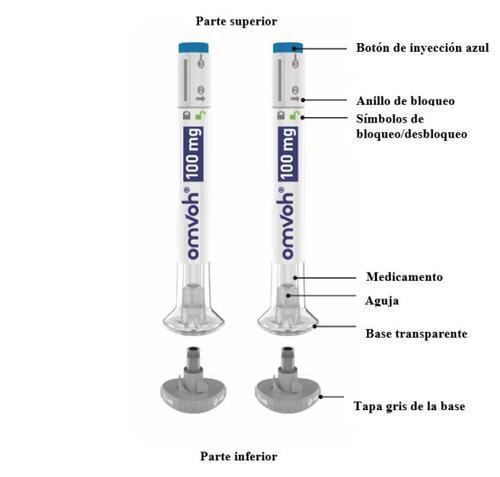
100mg+100mg=1 dosis completa
IMPORTANTE:
|
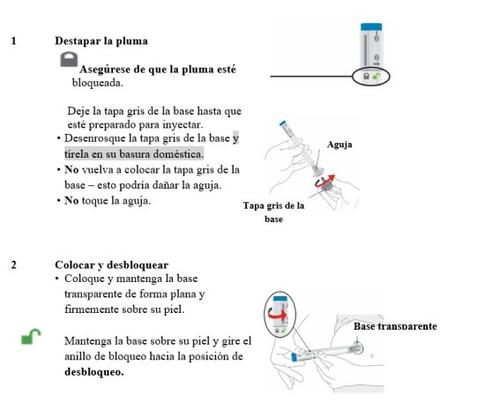
Preparación para inyectar Omvoh
Saque las plumas de la nevera | Saque 2 plumas de Omvoh de la nevera. Deje puestas las tapas grises de la base hasta que esté preparado para inyectar. Deje las plumas a temperatura ambiente durante 30 minutos antes de inyectar. Nocaliente las plumas en el microondas, no las moje con agua caliente, ni las exponga a la luz solar directa. Nouse las plumas si el medicamento está congelado. Noagitar. |
Reúna los elementos necesarios | Elementos necesarios:
|
Inspeccione las plumas y el medicamento
| Asegúrese de que tiene el medicamento correcto. El medicamento dentro debe ser transparente. El color puede variar de incoloro a ligeramente amarillo. Nouse las plumas, y elimínelas según las indicaciones de su profesional sanitario si:
|
Prepárese para inyectar | Lávese las manos con agua y jabón antes de inyectar Omvoh. |
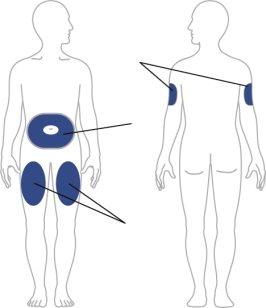
Elija su lugar de inyección
| Su profesional sanitario puede ayudarle a elegir el mejor lugar de inyección para usted.
Limpie el lugar de inyección con una toallita con alcohol. Deje que la zona donde se va a inyectar se seque antes de inyectar su medicamento. |
Inyectar Omvoh
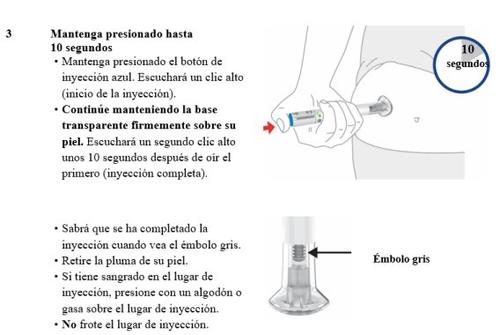
| |||
Tire las plumas de Omvoh | |||
Deseche las plumas usadas
|
|
- Si no tiene un contenedor para desechar objetos punzantes, puede usar un recipiente doméstico que sea:
- de plástico resistente,
- pueda cerrarse con una tapa hermética, resistente a los pinchazos, sin que puedan salir objetos cortantes,
- vertical y estable durante el uso,
- resistente a fugas,
- debidamente etiquetado para advertir de la presencia de residuos peligrosos en el interior del contenedor.
- Cuando su contenedor para desechar objetos punzantes esté casi lleno, deberá seguir las directrices de su comunidad sobre la forma correcta de deshacerse del mismo. Es posible que existan normativas locales sobre cómo se deben desechar las agujas y las plumas.
- No recicle su contenedor de eliminación de objetos punzantes usados.
- Para más información sobre cómo deshacerse del recipiente correctamente, pregunte a su profesional sanitario sobre las opciones disponibles en su zona.
Preguntas frecuentes
- ¿Qué pasa si dejo que mis plumas se atemperen durante más de 30 minutos antes de la inyección?
- Su pluma puede permanecer a temperatura ambiente hasta 30 ºC durante un máximo de 2 semanas.
- ¿Qué pasa si veo burbujas de aire en la pluma?
- Es normal que haya burbujas de aire en la pluma. No le perjudicarán ni afectarán a su dosis.
- ¿Qué pasa si hay una gota de líquido en la punta de la aguja cuando retiro la tapa gris de la base?
- Una gota de líquido en la punta de la aguja es normal. No le perjudicará ni afectará a su dosis.
- ¿Qué pasa si desbloqueo la pluma y presiono el botón de inyección azul hasta que se complete la inyección?
- No retire la tapa gris de la base. No utilice la pluma. Consulte con su médico o farmacéutico para obtener una nueva.
- ¿Necesito mantener presionado el botón de inyección azul hasta que se complete la inyección?
- No es necesario que mantenga presionado el botón de inyección azul, pero puede ayudarlo a mantener la pluma estable y firme sobre su piel.
- ¿Qué pasa si la aguja no se retrae después de mi inyección?
- No toque la aguja ni vuelva a colocar la tapa gris de la base. Guarde la pluma en un lugar seguro para evitar pinchazos accidentales y póngase en contacto con su médico, farmacéutico o enfermero.
- ¿Qué pasa si hay una gota de líquido o sangre en mi piel tras la inyección?
- Esto es algo normal. Presione con un algodón o gasa sobre el lugar de inyección. No frote el lugar de inyección.
- ¿Qué pasa si he oído más de 2 clics durante mi inyección - 2 clics fuertes y uno suave? ¿He recibido mi inyección completa?
- Algunos pacientes pueden escuchar un clic suave justo antes del segundo clic fuerte. Ese es el funcionamiento normal de la pluma. No retire la pluma de su piel hasta que escuche el segundo clic fuerte.
- ¿Cómo puedo saber si mi inyección está completa?
- Después de presionar el botón de inyección azul, escuchará 2 clics fuertes. El segundo clic fuerte le indica que su inyección está completa. También verá el émbolo gris en la parte superior de la base transparente.
Para conocer más sobre su medicamento, lea el prospecto completo de Omvoh dentro de este envase.
Última revisión en

- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a OMVOH 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 100 mgPrincipio activo: MirikizumabFabricante: Eli Lilly Nederland B.V.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 300 MGPrincipio activo: MirikizumabFabricante: Eli Lilly Nederland B.V.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 130 mgPrincipio activo: UstekinumabFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para OMVOH 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de OMVOH 100 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes