ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР
Спросите врача о рецепте на ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР

Инструкция по применению ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР
Введение
Прошпект: информация для пациента
Офлоксацин ПОС 3 мг/мл глазные капли в растворе
Прочитайте внимательно весь прошпект перед началом использования этого лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот прошпект, поскольку вам может потребоваться прочитать его снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено только вам, и не следует давать его другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этом прошпекте. См. раздел 4.
Содержание прошпекта
- Что такое Офлоксацин ПОС и для чего он используется
- Что вам нужно знать перед началом использования Офлоксацина ПОС
- Как использовать Офлоксацин ПОС
- Возможные побочные эффекты
- Хранение Офлоксацина ПОС
- Содержание упаковки и дополнительная информация
1. Что такое Офлоксацин ПОС и для чего он используется
Офлоксацин ПОС - это глазные капли, используемые для лечения наружных глазных инфекций, таких как конъюнктивит (воспаление конъюнктивы) и кератит (воспаление роговицы) у взрослых и детей, вызванных микроорганизмами, чувствительными к офлоксацину.
Офлоксацин ПОС относится к группе лекарств, называемых антибактериальными агентами типа 4-хинолонов.
Антибиотики используются для лечения бактериальных инфекций и не подходят для лечения вирусных инфекций.
Важно следовать инструкциям относительно дозы, интервала введения и продолжительности лечения, указанным вашим врачом.
Не храните и не повторно используйте это лекарство. Если после окончания лечения у вас осталось антибиотик, верните его в аптеку для правильной утилизации. Не следует выбрасывать лекарства в канализацию или мусор.
2. Что вам нужно знать перед началом использования Офлоксацина ПОС
Не используйте Офлоксацин ПОС
если вы аллергичны к офлоксацину, другим хинолонам или любому другому компоненту этого лекарства (указанному в разделе 6).
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед началом использования этого лекарства.
- если вы чувствительны к другим антибактериальным агентам типа хинолонов
- если у вас есть аномалии или язвы на поверхности глаза
Продолжительное использование может привести к новой бактериальной инфекции, которая может не поддаваться лечению Офлоксацином ПОС.
Во время лечения глазными каплями, содержащими офлоксацин, следует избегать чрезмерного воздействия солнечного света или ультрафиолетовых ламп (например, солнечных ламп, соляриев и т. д.) из-за потенциальной фотосенсибилизации.
Не рекомендуется использовать контактные линзы во время лечения глазной инфекции.
Были зарегистрированы случаи отека и разрыва сухожилий у людей, принимающих фторхинолоны перорально или внутривенно, особенно у пациентов пожилого возраста и у тех, кто одновременно лечится кортикостероидами. Прекратите принимать это лекарство, если у вас появляется боль или отек в сухожилиях (тендинит).
Будьте особенно осторожны с Офлоксацином ПОС
Прежде чем использовать Офлоксацин-ПОС
Проблемы с сердцем
Вам следует быть осторожным при использовании этого типа лекарства, если у вас есть врожденный или семейный анамнез продленного интервала QT (обнаруженного на ЭКГ, электрокардиограмме), если у вас есть нарушение баланса электролитов в крови (в основном низкие уровни калия или магния в крови), если у вас есть очень медленный сердечный ритм (брадикардия), если у вас есть ослабленное сердце (сердечная недостаточность), если у вас есть история сердечных приступов (инфаркта миокарда), если вы женщина или пациент пожилого возраста или принимаете другие лекарства, которые вызывают аномальные изменения ЭКГ (см. раздел Использование Офлоксацина ПОС с другими лекарствами).
Дети и подростки
Это лекарство может быть использовано у детей. Безопасность и эффективность не установлены у детей младше 1 года.
Использование Офлоксацина ПОС с другими лекарствами
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принимать любое другое лекарство.
Вам следует сообщить вашему врачу, если вы принимаете другие лекарства, которые могут изменить сердечный ритм: лекарства, принадлежащие к группе антиарритмиков (например, хинидин, гидрохинидин, дисопирамид, амиодарон, соталол, добетилид, ибутилид), трициклические антидепрессанты, некоторые антибиотики (принадлежащие к группе макролидов), некоторые антипсихотические лекарства.
Если вы используете другие глазные капли или мази кроме Офлоксацина ПОС, вы должны поддерживать интервал 15 минут между применением каждого лекарства. Глазные мази должны наноситься всегда в последнюю очередь.
Беременность и лактация
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства. Ваш врач оценит, может ли вы использовать Офлоксацин ПОС. Не рекомендуется использование Офлоксацина ПОС во время беременности.
Вождение и использование машин
Вы можете заметить размытое зрение сразу после применения Офлоксацина ПОС. Не驾驶айте и не используйте машины до тех пор, пока этот эффект не пройдет.
Офлоксацин ПОС содержит хлорид бензалкония
Это лекарство содержит 0,125 мг хлорида бензалкония в каждых 5 мл, что эквивалентно 0,025 мг/мл.
Хлорид бензалкония может быть абсорбирован мягкими контактными линзами и может изменить цвет контактных линз. Удалите контактные линзы перед использованием этого лекарства и подождите 15 минут перед повторным их применением.
Хлорид бензалкония может вызвать раздражение глаз, особенно если у вас сухость глаз или другие заболевания роговицы (прозрачной оболочки передней части глаза). Проконсультируйтесь с вашим врачом, если вы чувствуете странное ощущение, зуд или боль в глазу после использования этого лекарства.
3. Как использовать Офлоксацин ПОС
Следуйте точно инструкциям по применению этого лекарства, указанным вашим врачом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом.
Доза
Капните одну каплю Офлоксацина ПОС в пораженный глаз каждые два-четыре часа в течение первых двух дней и затем четыре раза в день. Чтобы Офлоксацин ПОС был эффективным, его необходимо использовать регулярно.
Способ применения
- Вымойте руки
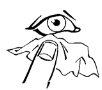
- Возьмите чистую ткань и осторожно протрите вокруг глаз, чтобы удалить любую влагу (диаграмма 1).
- Откройте бутылку и проверьте, что дозатор чист.
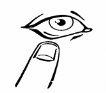
- Наклоните голову слегка назад и осторожно потяните вниз нижнее веко (диаграмма 2)
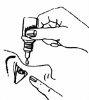
- и поместите 1-2 капли внутрь нижнего века (диаграмма 3). Будьте осторожны, чтобы не касаться глаза дозатором.
- Отпустите нижнее веко и осторожно нажмите внутренний угол глаза к носу (диаграмма 4). Затем, держа палец прижатым к носу, моргайте осторожно несколько раз, чтобы распределить каплю по поверхности глаза.
- Очистите любой излишек лекарства.
- Повторите этот процесс для другого глаза.
|
|
|
|
диаграмма 1 | диаграмма 2 | диаграмма 3 | диаграмма 4 |
Если капля попадает вне глаза, повторите операцию.
Чтобы избежать загрязнения, не допускайте, чтобы кончик дозатора касался вашего глаза или любой другой поверхности. Закройте бутылку и плотно закрутите крышку после использования.
Правильное применение капель в глаза очень важно. Если у вас есть какие-либо сомнения, проконсультируйтесь с вашим врачом или фармацевтом.
Продолжительность лечения
Продолжительность лечения не должна превышать 14 дней. Если симптомы сохраняются после этого времени, проконсультируйтесь с вашим врачом.
Использование у детей
Это лекарство может быть использовано у детей. Безопасность и эффективность не установлены у детей младше 1 года.
Если вы использовали больше Офлоксацина ПОС, чем следует
Если вы положили слишком много капель в глаз, промойте глаза водой. Затем нанесите следующую дозу, следуя обычной схеме.
В случае передозировки или случайного приема внутрь проконсультируйтесь с вашим врачом или фармацевтом или позвоните в Центр токсикологической информации, телефон: 91 562 04 20, указав лекарство и количество, принятое внутрь.
Если вы пропустили использование Офлоксацина ПОС
Если вы пропустили дозу, применьте ее как можно скорее, если только это не время следующей дозы, в этом случае пропустите пропущенную дозу.
Применьте следующую дозу и продолжайте обычную схему.
Не принимайте двойную дозу, чтобы компенсировать пропущенные дозы.
Если вы прекратите лечение Офлоксацином ПОС
Офлоксацин ПОС должен быть использован согласно указаниям вашего врача.
Если у вас есть какие-либо другие вопросы о использовании этого продукта, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Если возникает аллергическая реакция (включая воспаление кожи, слизистых оболочек, горла и/или языка, затруднение дыхания), прекратите введение глазных капель и немедленно обратитесь к вашему врачу.
Тяжелые побочные эффекты, частота неизвестна (не может быть оценена на основе доступных данных):
Были зарегистрированы случаи кожной сыпи (синдром Стивенса-Джонсона, токсический эпидермальный некролиз), связанные с использованием глазных капель с офлоксацином, которые могут быть потенциально смертельными, возникающими в виде красных пятен или круглых пятен, часто с пузырями в центре на теле.
Глазное раздражение и дискомфорт в глазах - это частые побочные эффекты (могут возникать у до 1 из 10 человек).
Следующие побочные эффекты могут возникнуть, хотя частота их возникновения неизвестна.
Побочные эффекты, влияющие на глаз:
- покраснение глаза/аллергия глаза
- зуд
- размытое зрение
- увеличение слезотечения
- сухость глаз
- боль в глазу
- чувствительность к свету
- воспаление вокруг глаз (включая воспаление век)
Побочные эффекты, влияющие на тело:
- аллергическая реакция
- головокружение
- онемение
- тошнота
- головная боль
- отек лица
Проблемы с сердцем:
Частота неизвестна: аномальный сердечный ритм, опасный для жизни аномальный сердечный ритм, нарушение сердечного ритма (называемое "продлением интервала QT", обнаруженным на ЭКГ, электрокардиограмме).
Сообщение о побочных эффектах:
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не указаны в этом прошпекте. Вы также можете сообщить о них напрямую через систему фармаковигиланса лекарств для человека: https://www.notificaram.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение Офлоксацина ПОС
Храните это лекарство в недоступном для детей месте.
Не используйте это лекарство после даты истечения срока годности, указанной на этикетке и упаковке после "Срок годности". Срок годности - последний день месяца, указанного.
Утилизируйте это глазное лекарство через 4 недели после первого открытия, даже если в нем еще осталась жидкость.
Не храните при температуре выше 25°C.
Храните упаковку в оригинальной внешней упаковке, чтобы защитить ее от света.
Если раствор приобретает более сильный желто-зеленый оттенок, этот продукт должен быть утилизирован.
Лекарства не должны быть выброшены в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в специальный пункт приема лекарств в аптеке. Если у вас есть сомнения, проконсультируйтесь с вашим фармацевтом, как правильно утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Офлоксацина ПОС
Активное вещество - офлоксацин 3 мг/мл.
Другие компоненты - хлорид бензалкония, хлорид натрия, соляная кислота и гидроксид натрия (регулирование pH), вода для инъекций.
Внешний вид продукта и содержание упаковки
Офлоксацин ПОС - это прозрачный раствор, бесцветный или слегка желто-зеленый, и выпускается в пластиковой бутылке с дозатором и винтовой крышкой.
Каждая упаковка содержит 1 пластиковую бутылку с 5 мл раствора.
Владелец разрешения на продажу и производитель
Владелец разрешения на продажу
BRILL PHARMA, S.L.
Улица Муннер, 8
08022 Барселона
Испания
Производитель
URSAPHARM Arzneimittel GmbH,
Промышленная улица,
66129 Саарбрюккен,
Германия
Это лекарство разрешено к продаже в государствах-членах Европейского экономического пространства под следующими названиями:
CZ Офлоксацин-ПОС 3мг/мл глазные капли, раствор
DE Офлоксацин-ПОС 3 мг/мл глазные капли, раствор
IT ВизуАб, глазные капли, раствор
ES Офлоксацин ПОС 3 мг/мл глазные капли в растворе
NL Офлоксацин-ПОС 3 мг/мл глазные капли, раствор
PL Офлоксацин-ПОС
Дата последнего пересмотра этого прошпекта:июнь 2020
Подробная и актуальная информация о этом лекарстве доступна на сайте Агентства по лекарствам и медицинским изделиям Испании (AEMPS) http://www.aemps.gob.es/

Сколько стоит ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР в Испании в 2026 году?
Средняя цена на ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР в январь, 2026 года составляет около 2.83 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках2.83 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОРФорма выпуска: ГЛАЗНЫЕ КАПЛИ, 30 мг/млАктивное вещество: ОфлоксацинПроизводитель: Abbvie Spain, S.L.U.Требуется рецептФорма выпуска: ГЛАЗНЫЕ КАПЛИ, 5 мг/млАктивное вещество: моксифлоксацинПроизводитель: Tiedra Farmaceutica S.L.Требуется рецептФорма выпуска: ГЛАЗНЫЕ КАПЛИ, 3 мг/млАктивное вещество: ЦипрофлоксацинПроизводитель: Laboratorios Salvat S.A.Требуется рецепт
Аналоги ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР в Польша
Аналог ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР в Украина
Врачи онлайн по ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ОФЛОКСАЦИН ПОС 3 мг/мл ГЛАЗНЫЕ КАПЛИ, РАСТВОР – по решению врача и с учетом местных правил.