NGENLA 24 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar NGENLA 24 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Ngenla 24mg solución inyectable en pluma precargada
somatrogón
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted o al niño bajo su cuidado, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted o el niño bajo su cuidado, ya que puede perjudicarles.
- Si usted o el niño bajo su cuidado experimentan efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ngenla y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ngenla
- Cómo usar Ngenla
- Posibles efectos adversos
- Conservación de Ngenla
- Contenido del envase e información adicional
1. Qué es Ngenla y para qué se utiliza
Ngenla contiene el principio activo somatrogón, una forma farmacéutica modificada de la hormona del crecimiento humana. La hormona del crecimiento humana natural es necesaria para que los huesos y los músculos crezcan. También ayuda a que la grasa y los tejidos musculares se desarrollen en las cantidades adecuadas. Ngenla se utiliza para tratar a niños y adolescentes a partir de los 3 años que no tienen suficiente hormona del crecimiento y no están creciendo al ritmo normal.
El principio activo de Ngenla se elabora mediante “tecnología de ADN recombinante”. Esto significa que se crea en células que se han modificado en el laboratorio para que puedan producirlo.
2. Qué necesita saber antes de empezar a usar Ngenla
No use Ngenla
- Si usted o el niño bajo su cuidado son alérgicos a somatrogón (ver “Advertencias y precauciones”) o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si usted o el niño bajo su cuidado tienen un tumor activo (cáncer). Informe a su médico si usted o el niño bajo su cuidado tienen o han tenido un tumor activo. Los tumores deben estar inactivos y usted o el niño bajo su cuidado deben haber terminado su tratamiento antitumoral antes de comenzar el tratamiento con Ngenla.
- Si usted o el niño bajo su cuidado han dejado de crecer debido al cierre de las placas de crecimiento (epífisis cerradas), lo que significa que su médico les ha dicho a usted o al niño bajo su cuidado que sus huesos han dejado de crecer.
- Si usted o el niño bajo su cuidado están gravemente enfermos (por ejemplo, sufren complicaciones después de una intervención quirúrgica a corazón abierto, cirugía abdominal, insuficiencia respiratoria aguda, traumatismos accidentales múltiples o afecciones similares). Si usted o el niño bajo su cuidado están a punto de someterse o se han sometido a una intervención quirúrgica importante, o van a ir al hospital por cualquier motivo, informe a su médico y recuerde a los otros médicos que les atienden que reciben hormona del crecimiento.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Ngenla:
- Si usted o el niño bajo su cuidado presentan una reacción alérgica grave, deje de usar Ngenla y consulte a su médico inmediatamente. En ocasiones, se han producido reacciones alérgicas graves como hipersensibilidad, que incluyen anafilaxia o angioedema (dificultades para respirar o tragar, o hinchazón de la cara, labios, garganta o lengua). Si usted o el niño bajo su cuidado tienen alguno de los siguientes síntomas de una reacción alérgica grave:
- Problemas respiratorios.
- Hinchazón de la cara, boca y lengua.
- Habones (urticaria, bultos que se elevan debajo de la piel).
- Erupción.
- Fiebre.
- Si usted o el niño bajo su cuidado reciben tratamiento sustitutivo con corticosteroides (glucocorticoides), debe consultar a su médico con regularidad, ya que usted o el niño bajo su cuidado pueden necesitar un ajuste de su dosis de glucocorticoides.
- Su médico debe comprobar de vez en cuando si la glándula tiroides funciona correctamente en usted o el niño bajo su cuidado y, si es necesario, podría recetarles un tratamiento o ajustar la dosis del tratamiento existente, ya que esto puede ser necesario para que Ngenla actúe correctamente.
- Si usted o el niño bajo su cuidado sufren síndrome de Prader‑Willi, usted o el niño no deben ser tratados con Ngenla a menos que tengan deficiencia de la hormona del crecimiento.
- Su médico debe vigilar la aparición en usted o el niño bajo su cuidado de niveles altos de azúcar en sangre (hiperglucemia) durante el tratamiento con Ngenla. Si usted o el niño bajo su cuidado reciben tratamiento con insulina u otros medicamentos para la diabetes, es posible que su médico deba ajustar la dosis de insulina. Si usted o el niño bajo su cuidado tienen diabetes y una enfermedad ocular relacionada grave o que empeora, no deben recibir tratamiento con Ngenla.
- Si alguna vez usted o el niño bajo su cuidado han tenido algún tipo de tumor (cáncer).
- Si usted o el niño bajo su cuidado experimentan cambios en la visión, dolores de cabeza intensos o frecuentes, relacionados con náuseas, vómitos, o experimenta falta de control muscular o de coordinación de movimientos voluntarios, tales como andar o agarrar objetos, dificultad en el habla, el movimiento ocular o dificultad para tragar, especialmente al inicio del tratamiento, informe a su médico inmediatamente. Estos podrían ser signos de un aumento temporal de la presión dentro del cerebro (hipertensión intracraneal).
- Si usted o el niño bajo su cuidado están gravemente enfermos (por ejemplo, sufren complicaciones después de una intervención quirúrgica a corazón abierto, intervención quirúrgica abdominal, insuficiencia respiratoria aguda, traumatismos accidentales múltiples o afecciones similares). Si usted o el niño bajo su cuidado están a punto de someterse o se han sometido a una intervención quirúrgica importante, o van a ir al hospital por cualquier motivo, informe a su médico y recuerde a los otros médicos que les atienden que usted o el niño bajo su cuidado reciben hormona del crecimiento.
- Si usted o el niño bajo su cuidado presentan un dolor de estómago intenso durante el tratamiento con Ngenla, ya que podría ser un síntoma de inflamación del páncreas.
- Si usted o el niño bajo su cuidado notan una curvatura lateral en su columna vertebral (escoliosis), usted o el niño bajo su cuidado deberán ser examinados con frecuencia por su médico.
- Si durante el crecimiento, usted o el niño bajo su cuidado presentan cojera o dolor en la cadera o la rodilla, usted o el niño bajo su cuidado deben consultar a su médico inmediatamente. Estos podrían ser síntomas de trastornos óseos en la cadera, ya que esto puede ocurrir durante períodos de rápido crecimiento.
- Si usted o el niño bajo su cuidado están tomando o dejan de tomar anticonceptivos orales o tratamiento sustitutivo hormonal con estrógenos, su médico puede recomendar que se ajuste la dosis de Ngenla.
Otros medicamentos y Ngenla
Informe a su médico, farmacéutico o enfermero si usted o el niño bajo su cuidado están utilizando, han utilizado recientemente o pudieran tener que utilizar cualquier otro medicamento.
- Si usted o el niño bajo su cuidado reciben tratamiento sustitutivo con corticosteroides (glucocorticoides), ya que estos pueden reducir el efecto de Ngenla sobre el crecimiento. Usted o el niño bajo su cuidado deben consultar a su médico con regularidad, ya que usted o el niño bajo su cuidado pueden necesitar un ajuste de su dosis de glucocorticoides.
- Si usted o el niño bajo su cuidado reciben tratamiento con insulina u otros medicamentos para la diabetes, deben consultar a su médico, ya que es posible que usted o su médico deban ajustar la dosis.
- Si usted o el niño bajo su cuidado están recibiendo tratamiento con hormonas tiroideas, es posible que su médico deba ajustar la dosis.
- Si usted o el niño bajo su cuidado está recibiendo estrógenos por vía oral, debe consultar a su médico, ya que es posible que usted o el niño necesiten ajustar su dosis de Ngenla.
- Si usted o el niño bajo su cuidado está recibiendo ciclosporina (un medicamento que debilita el sistema inmunitario después de un trasplante), debe consultar a su médico, ya que es posible que su médico deba ajustar la dosis.
- Si usted o el niño bajo su cuidado está recibiendo medicamentos para controlar la epilepsia (anticonvulsivos), debe consultar a su médico, ya que es posible que su médico deba ajustar la dosis.
Embarazo y lactancia
Si usted o la niña bajo su cuidado están embarazadas o en periodo de lactancia, cree que usted o la niña bajo su cuidado podrían estar embarazadas o tienen intención de quedarse embarazadas, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Ngenla no se ha probado en mujeres embarazadas y no se sabe si este medicamento puede dañar al feto. Por tanto, es preferible evitar Ngenla durante el embarazo. Si puede quedarse embarazada, no debe utilizar Ngenla a menos que también esté utilizando un método anticonceptivo fiable.
Se desconoce si somatrogón puede pasar a la leche materna. Informe a su médico o al médico de la niña bajo su cuidado si usted o la niña bajo su cuidado están dando el pecho o planean hacerlo. Su médico le ayudará a decidir si usted o la niña bajo su cuidado deben interrumpir la lactancia o dejar de recibir Ngenla, teniendo en cuenta el beneficio de la lactancia para el bebé y el beneficio de Ngenla para usted o la niña bajo su cuidado.
Conducción y uso de máquinas
Ngenla no afecta a la capacidad para conducir y utilizar máquinas.
Ngenla contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
Ngenla contiene metacresol
Ngenla contiene un conservante llamado metacresol. En casos muy raros, la presencia de metacresol puede causar inflamación (hinchazón) en los músculos. Si usted o el niño bajo su cuidado experimentan dolor muscular o dolor en la zona de inyección, informen a su médico.
3. Cómo usar Ngenla
Este medicamento solo lo recetará un médico con experiencia en el tratamiento con hormona del crecimiento y que haya confirmado su diagnóstico o el del niño bajo su cuidado.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Su médico decidirá la dosis de Ngenla que debe inyectarse.
Cuánto usar
Su médico calculará su dosis de Ngenla a partir de su peso corporal en kilogramos. La dosis recomendada es de 0,66 mg por kg de peso corporal y se administra una vez a la semana. Si usted o el niño bajo su cuidado han sido tratados previamente con inyecciones diarias de hormona del crecimiento, su médico les indicará que esperen antes de recibir la primera dosis de Ngenla hasta el día después de su última inyección diaria y que luego continúen con Ngenla una vez a la semana.
No cambie su dosis a menos que su médico se lo indique.
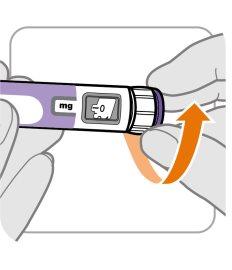
Cómo se administra Ngenla
- Ngenla está disponible como pluma precargada en 2 tamaños diferentes (Ngenla 24 mg y Ngenla 60 mg). Según la dosis recomendada, su médico o el médico del niño bajo su cuidado le recetará el tamaño de pluma más adecuado (ver sección 6 “Contenido del envase e información adicional”).
- Antes de que usted o el niño bajo su cuidado usen la pluma por primera vez, su médico o enfermero les enseñará cómo usarla. Ngenla se administra mediante una inyección debajo de la piel (inyección subcutánea) utilizando una pluma precargada. No lo inyecte en una vena o músculo.
- La mejor zona para administrar Ngenla es el abdomen (vientre), los muslos, las nalgas y la parte superior de los brazos. Las inyecciones en la parte superior de los brazos y las nalgas deben ser administradas por el cuidador.
- Cambie la zona de inyección en su cuerpo o el cuerpo del niño bajo su cuidado cada vez que se administre una dosis.
- Si se requiere más de una inyección para administrar una dosis completa, cada una debe administrarse en una zona de inyección diferente.
Las instrucciones de uso detalladas de la pluma precargada se encuentran al final de este prospecto.
Cuándo utilizar Ngenla
Usted o el niño bajo su cuidado deben utilizar este medicamento una vez a la semana el mismo día de cada semana.
Usted o el niño bajo su cuidado deben registrar qué día de la semana utilizan Ngenla para ayudarles a recordar inyectarse este medicamento una vez a la semana.
Si es necesario, usted o el niño bajo su cuidado pueden cambiar el día de su inyección semanal siempre que hayan pasado al menos 3 días desde que usted o el niño bajo su cuidado recibieron la última inyección. Después de seleccionar un nuevo día de administración, continúe administrándose la inyección a sí mismo o al niño bajo su cuidado ese día cada semana.
Si usa más Ngenla del que debe
Si usted o el niño bajo su cuidado se han inyectado más Ngenla del que deben, póngase en contacto con su médico inmediatamente, ya que puede ser necesario controlar sus niveles de azúcar en sangre.
Si olvidó usar Ngenla
Si usted o el niño bajo su cuidado olvidaron inyectarse una dosis y:
- Han pasado 3 días o menos desde que usted o el niño bajo su cuidado deberían haber usado Ngenla, úsenlo tan pronto como lo recuerden. Luego, inyecten su siguiente dosis el día de la inyección habitual.
- Han pasado más de 3 días desde que usted o el niño bajo su cuidado deberían haber usado Ngenla, sáltense la dosis olvidada. Luego, inyecten su siguiente dosis como de costumbre el próximo día programado. Se debe mantener un día de administración habitual.
No use una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con Ngenla
No deje de usar este medicamento sin consultar a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes: pueden afectar a más de 1 de cada 10personas
- Dolor de cabeza
- Sangrado, inflamación, picazón, dolor, enrojecimiento, molestias, escozor, sensibilidad o calor en la zona de inyección (reacciones en la zona de inyección)
- Fiebre (pirexia)
Frecuentes: pueden afectar hasta 1 de cada 10personas
- Disminución del número de glóbulos rojos en sangre (anemia)
- Aumento del número de eosinófilos en sangre (eosinofilia)
- Disminución del nivel sanguíneo de hormona tiroidea (hipotiroidismo)
- Inflamación alérgica de la conjuntiva, la capa transparente que recubre el exterior del ojo (conjuntivitis alérgica)
- Dolor en las articulaciones (artralgia)
- Dolor en brazos o piernas
Poco frecuentes: pueden afectar hasta1 de cada 100personas
- Las glándulas suprarrenales no producen suficientes hormonas esteroides (insuficiencia suprarrenal)
- Erupción
Otros posibles efectos adversosno observados conNgenlapero que han sidonotificadoscon otros tratamientos medicinales dehormona del crecimiento pueden incluir los siguientes:
- Crecimiento de tejidos (no cancerosos o cancerosos)
- Diabetes de tipo 2
- Aumento de la presión intracraneal (que provoca síntomas como fuerte dolor de cabeza, alteraciones visuales o vómitos)
- Entumecimiento u hormigueo
- Dolor en las articulaciones o músculos
- Aumento de las mamas en niños y hombres
- Erupción cutánea, enrojecimiento y picor
- Retención de agua (que se manifiesta como dedos hinchados o tobillos hinchados)
- Hinchazón de la cara
- Pancreatitis (que causa síntomas de dolor de estómago, náuseas, vómitos o diarrea)
En casos muy raros, la presencia de metacresol puede causar inflamación (hinchazón) en los músculos. Si usted o el niño bajo su cuidado experimentan dolor muscular o dolor en la zona de inyección, informe a su médico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ngenla
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la pluma o en la caja después de “EXP”. La fecha de caducidad es el último día del mes que se indica.
La pluma precargada no se debe utilizar más de 28 días después del primer uso.
Antes del primer uso de Ngenla
- Conservar en nevera (entre 2 °C y 8 °C).
- Mantener Ngenla en el embalaje exterior para protegerlo de la luz.
- Retire Ngenla de la nevera antes de su uso. Ngenla puede mantenerse a temperatura ambiente (hasta un máximo de 32 °C) hasta un máximo de 4 horas.
- No utilice este medicamento si observa que la solución está turbia o de color amarillo oscuro. No utilice el medicamento si tiene escamas o partículas.
- No agite la pluma. Agitarla puede dañar la medicación.
Después del primer uso de Ngenla
- Úselo dentro de los 28 días posteriores al primer uso. Conservar en nevera (entre 2 °C y 8 °C). No congelar.
- Mantenga Ngenla con el capuchón de la pluma puesto para protegerlo de la luz.
- No guarde la pluma precargada con la aguja puesta.
- Deseche la pluma después de la última dosis, incluso si contiene medicamento sin usar.
- Ngenla puede mantenerse a temperatura ambiente (hasta un máximo de 32 °C) hasta un máximo de 4 horas con cada inyección, hasta un máximo de 5 veces. Vuelva a colocar Ngenla en la nevera después de cada uso.
- No la deje a temperatura ambiente durante más de 4 horas con cada uso.
- No coloque la pluma en ningún lugar donde la temperatura supere los 32 °C.
- Si han pasado más de 28 días desde el primer uso de la pluma, deshágase de ella aunque contenga medicamento sin usar. Si su pluma o la pluma del niño bajo su cuidado han estado expuestas a temperaturas superiores a 32 °C, se han sacado de la nevera durante más de 4 horas con cada uso o se han utilizado un total de 5 veces, deshágase de ellas incluso si contienen medicamento sin usar.
Para ayudarle a recordar cuándo desechar su pluma, puede escribir la fecha del primer uso en la etiqueta de la pluma.
Es posible que quede una pequeña cantidad de medicamento en la pluma después de que se hayan administrado correctamente todas las dosis. No intente usar el medicamento restante. Después de administrar la última dosis, la pluma se debe desechar correctamente.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ngenla
- El principio activo es somatrogón.
Ngenla 24 mg solución inyectable en pluma precargada
Un ml de solución contiene 20 mg de somatrogón.
Cada pluma precargada contiene 24 mg de somatrogón en 1,2 ml de solución. Cada pluma precargada proporciona dosis de 0,2 mg a 12 mg en una sola inyección en incrementos de 0,2 mg.
Ngenla 60 mg solución inyectable en pluma precargada
Un ml de solución contiene 50 mg de somatrogón.
Cada pluma precargada contiene 60 mg de somatrogón en 1,2 ml de solución. Cada pluma precargada proporciona dosis de 0,5 mg a 30 mg en una sola inyección en incrementos de 0,5 mg.
- Los demás componentes son: citrato trisódico dihidrato, ácido cítrico monohidrato, L-histidina, cloruro de sodio (ver sección 2 “Ngenla contiene sodio”), poloxámero 188, m-Cresol y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Ngenla es una solución inyectable (inyectable) transparente y de incolora a ligeramente amarilla clara en una pluma precargada.
Ngenla 24 mg solución inyectable está disponible en un tamaño de envase que contiene 1 pluma precargada. El capuchón de la pluma, el botón dosificador y la etiqueta de la pluma son de color lila.
Ngenla 60 mg solución inyectable está disponible en un tamaño de envase que contiene 1 pluma precargada. El capuchón de la pluma, el botón dosificador y la etiqueta de la pluma son de color azul.
Titular de la autorización de comercialización Pfizer Europe MA EEIG Boulevard de la Plaine 17 1050 Bruxelles Bélgica |
Responsable de la fabricación Pfizer Manufacturing Belgium NV Rijksweg 12 2870 Puurs-Sint-Amands Bélgica |
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tél/Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel: +370 5 251 4000 |
???????? ??????? ?????????? ????, ???? ???????? ???.: +359 2 970 4333 | Magyarország Pfizer Kft. Tel.: + 36 1 488 37 00 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +356 21344610 |
Danmark Pfizer ApS Tlf.: +45 44 20 11 00 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Ελλ?δα Pfizer Ελλ?ς Α.Ε. Τηλ: +30 210 6785800 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
España Pfizer S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L. Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: + 386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: 1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Ísland Icepharma hf. Sími: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Κ?προς Pfizer Ελλ?ς Α.Ε. (Cyprus Branch) Τηλ: +357 22817690 | |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel: +371 670 35 775 |
Fecha de la última revisión de este prospecto 04/2025.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
Instrucciones de uso
Pluma Ngenla de 24 mg
Inyección solo para uso subcutáneo (debajo de la piel)
Conserve este prospecto. Estas instrucciones indican paso a paso
cómo preparar y administrar una inyección de Ngenla.
Información importante sobre la pluma Ngenla
- Ngenla inyectable es una pluma precargada multidosis que contiene 24 mg de medicamento.
- Ngenla inyectable puede ser administrado por un paciente, un cuidador, un médico, un enfermero o un farmacéutico. Nointente inyectarse Ngenla usted mismo hasta que le muestren la forma correcta de administrar las inyecciones y lea y comprenda las Instrucciones de Uso. Si su médico, enfermero o farmacéutico decide que usted o un cuidador pueden administrar las inyecciones de Ngenla en casa, debe recibir formación sobre la forma correcta de preparar e inyectar Ngenla. Es importante que consulte con su médico, enfermero o farmacéutico para asegurarse de que comprende las instrucciones de administración de Ngenla.
- Para ayudarle a recordar cuándo inyectarse Ngenla, puede marcar su calendario con antelación. Llame a su médico, enfermero o farmacéutico si usted o su cuidador tienen alguna pregunta sobre la forma correcta de inyectar Ngenla.
- Cada giro (clic) del botón dosificador aumenta la dosis en 0,2 mg de medicamento. Puede administrar de 0,2 mg a 12 mg en una sola inyección. Si su dosis es superior a 12 mg, deberá administrar más de 1 inyección.
- Puede que una pequeña cantidad del medicamento permanezca en la pluma después de que todas las dosis se hayan administrado correctamente. Esto es normal. Los pacientes no deben intentar usar la solución restante, sino deshacerse de la pluma de la forma correcta.
- Nocomparta su pluma con otras personas, incluso si se ha cambiado la aguja. Puede contagiar a otras personas una infección grave o contraer una infección grave de ellas.
- Utilice siempre una nueva aguja estéril para cada inyección. Esto reducirá el riesgo de contaminación, infección, pérdidas de medicamento y agujas bloqueadas que conduzcan a una dosis incorrecta.
- Noagite la pluma. Agitar la pluma puede dañar el medicamento.
- No se recomiendael uso de la pluma por personas ciegas o con deficiencia visual sin la ayuda de una persona formada en el uso adecuado del producto.
Materiales que necesitará cada vez que se inyecte
Incluido en la caja:
- 1 pluma Ngenla de 24 mg.
No incluido en la caja:
- 1 aguja estéril nueva para cada inyección.
- Toallitas impregnadas en alcohol.
- Torundas de algodón o compresas de gasa.
- Apósito adhesivo.
- Un recipiente para objetos punzocortantes adecuado para la eliminación de las agujas de las plumas y las plumas.
Pluma Ngenla de 24mg:
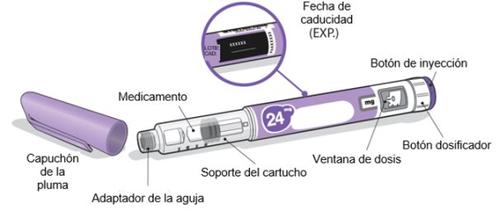
Agujas que se pueden usar
Las agujas de la pluma no se incluyencon la pluma Ngenla. Puede utilizar agujas para plumas de 4 mm a 8 mm y entre 30G y 32G.
- Se ha demostrado que las siguientes agujas son compatibles con la pluma Ngenla:
- 32G (Novo Nordisk®, NovoFine® Plus)
- 31G (Novo Nordisk®, NovoFine®)
- 31G (Becton Dickinson and Company, BD Ultra-Fine™ o BD Micro-Fine™)
- Se ha demostrado que las siguientes agujas de seguridad son compatibles con la pluma Ngenla:
- 30G (Becton Dickinson and Company, AutoShield Duo™)
- 30G (Novo Nordisk®, NovoFine® AutoCover®)
- Consulte a su médico, enfermero o farmacéutico sobre la aguja adecuada para usted.
Aguja estéril (ejemplo) no incluida:
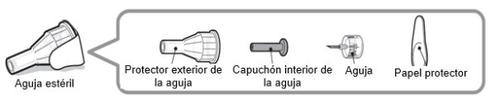
Nota:Las agujas de seguridad no tienen un capuchón interior de la aguja. Es posible que los pasos 5, 6 y 11 de estas instrucciones relativos al capuchón interior de la aguja no sean aplicables cuando se utiliza una aguja de seguridad. Consulte las instrucciones de uso del fabricante de la aguja para obtener más información.
Precaución:Nunca use una aguja doblada o dañada. Siempre manipule las agujas de las plumas con cuidado para asegurarse de no pincharse a sí mismo (ni a nadie más) con la aguja. Nocoloque una nueva aguja en la pluma hasta que esté listo para la inyección.
Preparación para la inyección
Paso1 Preparación
- Lávese y séquese las manos.
- Puede utilizar su pluma directamente de la nevera. Para una inyección más cómoda, deje su pluma a temperatura ambiente hasta un máximo de 30 minutos. (Ver sección5 “Conservación de Ngenla” del prospecto de la pluma precargada Ngenla de 24mg).
- Compruebe el nombre, la concentración y la etiqueta de la pluma para asegurarse de que sea el medicamento que su médico le ha recetado.
- Compruebe la fecha de caducidad en la etiqueta de la pluma. Nola use si ha pasado la fecha de caducidad.
- Nouse la pluma si:
- Se ha congelado o expuesto al calor (por encima de 32 ºC) o han pasado más de 28 días desde el primer uso de la pluma. (Ver sección5 “Conservación de Ngenla” del prospecto de la pluma precargada Ngenla de 24mg).
- Se ha caído.
- Parece rota o dañada.
- Noretire el capuchón de la pluma hasta que esté listo para la inyección.
Paso2Elija y limpie la zona de inyección
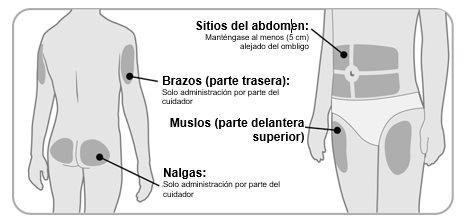
- Ngenla se puede administrar en el abdomen (vientre), los muslos, las nalgas o la parte superior de los brazos.
- Elija la mejor zona para inyección, según lo recomiende su médico, enfermero o farmacéutico.
- Si se necesita más de 1 inyección para completar su dosis, cada inyección se debe administrar en una zona de inyección diferente.
- Noinyecte en zonas de hueso, zonas magulladas, enrojecidas, doloridas o duras o zonas que tengan cicatrices o afecciones de la piel.
- Limpie la zona de inyección con una toallita impregnada en alcohol.
- Deje que se seque la zona de inyección.
- Notoque la zona de inyección después de limpiarlo.
Paso3 Compruebe el medicamento
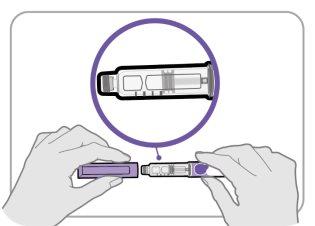
- Quite el capuchón de la pluma y guárdelo para después de la inyección.
- Compruebe el medicamento dentro del soporte del cartucho.
- Asegúrese de que el medicamento sea transparente y entre incoloro y ligeramente amarillo claro. Noinyecte el medicamento si está turbio o de color amarillo oscuro.
- Asegúrese de que el medicamento esté libre de escamas o partículas. Noinyecte el medicamento si tiene escamas o partículas.
Nota:Es normal ver una o más burbujas en el medicamento.
Paso4Coloquela aguja
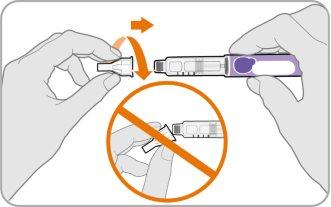
- Coja una aguja nueva y retire el papel protector.
- Alinee la aguja con su pluma manteniéndolos rectos.
- Empuje suavemente y luego enrosque la aguja en su pluma.
Noapriete demasiado.
Nota:Tenga cuidado de no colocar la aguja en ángulo. Esto puede hacer que la pluma gotee.
Precaución:Las agujas tienen puntas afiladas en ambos extremos. Tenga precaución para asegurarse de no pincharse a sí mismo (ni a nadie más) con la aguja.
Paso5 Retire el protector exterior de la aguja
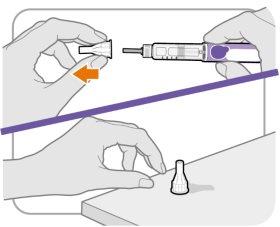
- Retire el protector exterior de la aguja.
- Asegúrese de conservar el protector exterior de la aguja. Lo necesitará más tarde para quitar la aguja.
Nota:Debería ver un capuchón interior de la aguja después de haber quitado el protector exterior. Si no ve esto, intente colocar la aguja de nuevo.
Nota:Si utiliza una aguja de seguridad, consulte las instrucciones de uso del fabricante.
Paso6 Retire el capuchón interior de la aguja
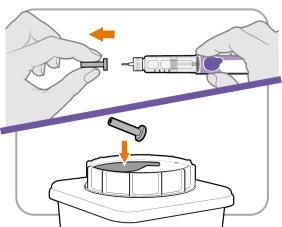
- Retire el capuchón interior de la aguja con cuidado para mostrar la aguja.
- Deseche el capuchón interior de la aguja en un recipiente para objetos punzocortantes. Ya no es necesario.
Nota:Si utiliza una aguja de seguridad, consulte las instrucciones de uso del fabricante.
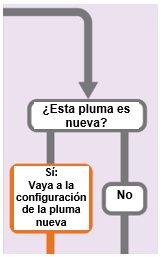
(“Sí:Vaya a configuración de pluma nueva” tiene una flecha que indica “Configuración de una pluma nueva (cebado)” y “No” tiene una flecha que indica “Ajuste su dosis prescrita”)
Configuración de una pluma nueva (cebado): solo para el primer uso de una pluma nueva
Debe configurar cada pluma nueva (cebado) antes de usarla por primera vez
- La configuración de la pluma nueva se realiza antes de que cada pluma nueva se utilice por primera vez.
- El propósito de configurar una pluma nueva es eliminar las burbujas de aire y asegurarse de que recibe la dosis correcta.
Importante:Salte del Paso A al Paso C si ya ha configurado la pluma.
Paso‑A: Ponga el botón en0,4
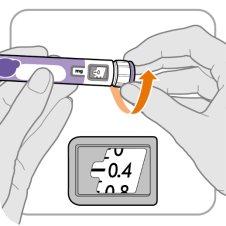
- Gire el botón dosificador a 0,4.
Nota:Si gira el botón dosificador demasiado, puede volver atrás.
Paso‑B: Golpee el soporte del cartucho
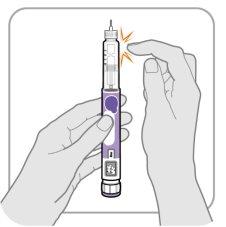
- Sostenga la pluma con la aguja apuntando hacia arriba para que las burbujas de aire puedan subir.
- Golpeesuavemente el soporte del cartucho para hacer flotar las burbujas de aire hacia la parte superior.
Importante:Siga el Paso B incluso si no ve burbujas de aire.
Paso‑C: Presione el botón y compruebe si hay líquido
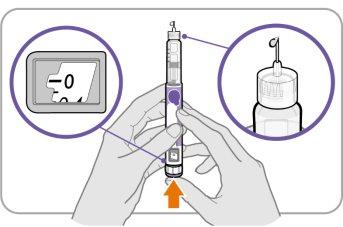
- Presione el botón de inyecciónhasta que no pueda avanzar más y aparezca “0”en la ventana de dosis.
- Compruebesi hay líquido en la punta de la aguja. Si aparece líquido, su pluma está configurada.
- Asegúrese siempre de que aparezca una gota de líquido antes de la inyección. Si no ha aparecido líquido, repita del Paso A al Paso C.
- Si no aparece líquido después de haber repetido del Paso A al Paso C cinco (5) veces, coloque una nueva aguja e inténtelo una (1) vez más.
Noutilice la pluma si aún no aparece una gota de líquido. Contacte con su médico, enfermero o farmacéutico y use una pluma nueva.
Ajuste su dosis prescrita
Paso7 Ajuste su dosis
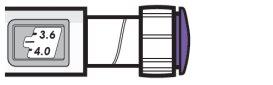
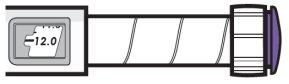
| Ejemplo A
3,8 mg se muestran en la ventana de dosis Ejemplo B
12,0 mg se muestran en la ventana de dosis |
- Gire el botón dosificador para ajustar su dosis.
- La dosis se puede aumentar o disminuir girando el botón dosificador en cualquier dirección.
- El botón dosificador gira 0,2 mg cada vez.
- Su pluma contiene 24 mg de medicamento, pero solo puede ajustar una dosis de hasta 12 mg para una sola inyección.
- La ventana de dosis muestra la dosis en mg. Ver ejemplosA yB.
- Siempre compruebe la ventana de dosis para asegurarse de haber ajustado la dosis correcta.
Importante:Nopresione el botón de inyección mientras ajuste su dosis.
¿Qué debo hacer si no puedo ajustar la dosis que necesito?
- Si su dosis es superior a 12 mg, necesitará más de 1 inyección.
- Puede administrar de 0,2 mg a 12 mg en una sola inyección.
- Si necesita ayuda para dividir su dosis de la forma correcta, consulte a su médico, enfermero o farmacéutico.
- Utilice una aguja nueva para cada inyección (ver Paso4:Coloque la aguja).
- Si normalmente necesita administrar 2 inyecciones para su dosis completa, asegúrese de administrar su segunda dosis.
¿Qué debo hacer si no queda suficiente medicamento en mi pluma?
- Si su pluma contiene menos de 12 mg de medicamento, el botón dosificador se detendrá con la cantidad restante de medicamento que se muestra en la ventana de dosis.
- Si no queda suficiente medicamento en su pluma para su dosis completa, puede:
- Inyectar la cantidad restante en su pluma y preparar luego una nueva pluma para completar su dosis.
Recuerde restar la dosis que ya ha recibido. Por ejemplo, si la dosis es de 3,8 mg y solo puede ajustar el botón dosificador en 1,8 mg, debe inyectar otros 2,0 mg con una pluma nueva.
- O conseguir una pluma nueva e inyectar la dosis completa.
Inyecte su dosis
Paso8 Inserte la aguja
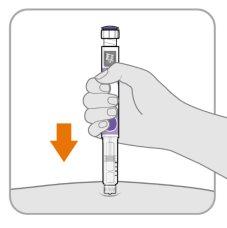
- Sostenga la pluma para que pueda ver los números en la ventana de dosis.
- Inserte la aguja directamente en la piel.
Paso9 Inyecte el medicamento
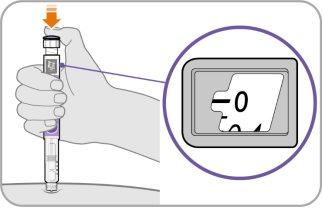
- Siga sosteniendo la aguja en la misma posición en su piel.
- Presione el botón de inyecciónhasta que no pueda avanzar más y aparezca “0”en la ventana de dosis.
Paso10 Cuente hasta10
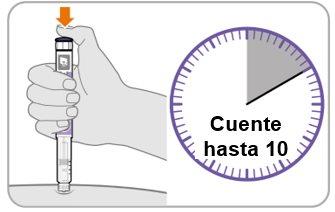
- Continúe presionando el botón de inyección mientras cuenta hasta10. Contar hasta 10 permitirá que se administre la dosis completa del medicamento.
- Después de contar hasta 10, suelte el botón de inyección y retire lentamente la pluma de la zona de inyección tirando de la aguja hacia afuera.
Nota:Es posible que vea una gota de medicamento en la punta de la aguja. Esto es normal y no afecta a la dosis que acaba de recibir.
Paso11 Coloque el protector exterior de la aguja
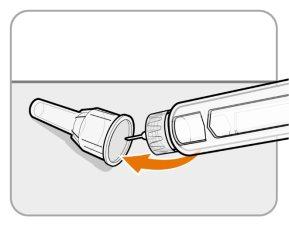
- Vuelva a colocar con cuidado el protector exterior de la aguja sobre la aguja.
- Presione el protector exterior de la aguja hasta que esté fijado.
Precaución:Nunca intente volver a colocar el capuchón interior de la aguja en la aguja. Puede pincharse con la aguja.
Nota:Si utiliza una aguja de seguridad, consulte las instrucciones de uso del fabricante.
Paso12 Retire la aguja
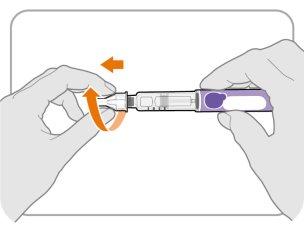
- Desenrosque la aguja de la pluma.
- Tire suavemente hasta que salga la aguja.
Nota:Si la aguja aún está puesta, vuelva a colocar el protector exterior de la aguja e inténtelo de nuevo. Asegúrese de aplicar presión al desenroscar la aguja.
Deseche las agujas de la pluma usadas en un recipiente para objetos punzocortantes según las instrucciones de su médico, enfermero o farmacéutico y de acuerdo con las normativas locales de salud y seguridad. Mantenga el recipiente para objetos punzocortantes fuera del alcance de los niños. Noreutilice las agujas.
Paso13 Vuelva a colocar el capuchón de la pluma
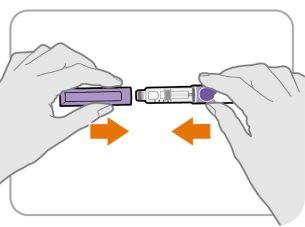
- Vuelva a colocar el capuchón de la pluma en la pluma.
- Novuelva a tapar la pluma con una aguja puesta.
- Si queda algo de medicamento en su pluma, guárdela en la nevera entre usos (ver sección5 “Conservación de Ngenla” del prospecto de la pluma precargada Ngenla de 24mg).
Paso14 Después de la inyección
- Presione ligeramente en la zona de inyección con una torunda de algodón o compresa de gasa y manténgala presionada durante unos segundos.
- Nofrote la zona de inyección. Es posible que tenga un sangrado leve. Esto es normal.
- Puede cubrir la zona de inyección con un pequeño apósito adhesivo, si es necesario.
- Si la pluma está vacía o han pasado más de 28díasdespués del primer uso, deséchela incluso si contiene medicamento sin usar. Deseche la pluma en el recipiente para objetos punzocortantes.
- Para ayudarle a recordar cuándo desechar la pluma, puede escribir la fecha del primer uso en la etiqueta de la pluma y a continuación:
Fecha del primer uso ______ / ______ / ______
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NGENLA 24 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 60 mg /1,2mlPrincipio activo: SomatrogonFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: INYECTABLE, 12 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 5,3 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere receta
Médicos online para NGENLA 24 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NGENLA 24 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes