NASACORT 55 microgramos/dosis, SUSPENSION PARA PULVERIZACION NASAL

Cómo usar NASACORT 55 microgramos/dosis, SUSPENSION PARA PULVERIZACION NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Nasacort 55 microgramos/dosis, suspensión para pulverización nasal
Acetónido de triamcinolona
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas de enfermedad que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Nasacort y para qué se utiliza
- Qué necesita saber antes de empezar a usar Nasacort
- Cómo usar Nasacort
- Posibles efectos adversos
5 Conservación de Nasacort
- Contenido del envase e información adicional
1. Qué es Nasacort y para qué se utiliza
Nasacort contiene un medicamento denominado acetónido de triamcinolona. Éste pertenece a un grupo de medicamentos conocidos como corticosteroides, lo que significa que es un tipo de esteroide. Se administra como un pulverizador en la nariz para tratar los síntomas nasalesde la rinitis alérgica en adultos y niños mayores de 2 años.
Los síntomas nasalesde alergia incluyen estornudos, picor y bloqueo, congestión o goteo nasal. Pueden estar provocados por:
- Pelo de animales o ácaros del polvo. Este tipo de alergia puede ocurrir en cualquier momento del año y se llama “rinitis alérgica perenne”.
- Polen. Este tipo de alergia, como la fiebre del heno, puede estar causada por distintos pólenes en diferentes estaciones del año. Se llama “rinitis alérgica estacional”.
Este medicamento sólo es eficaz si se emplea de forma regular y es posible que sus síntomas no mejoren de forma instantánea. Ayuda a algunas personas desde el primer día de tratamiento, sin embargo, en otras personas se puede tardar de 3 a 4 días en sentir una mejoría.
2. Qué necesita saber antes de empezar a usar Nasacort
No use Nasacort:
- si es alérgico al acetónido de triamcinolona o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Los síntomas de una reacción alérgica a Nasacort incluyen: erupción (urticaria), picor, problemas para tragar o para respirar, hinchazón de los labios, cara, garganta o lengua.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Nasacort:
- si usted sufre cualquier infección en la nariz o la garganta que no está siendo tratada. Si desarrolla una infección por hongos mientras utiliza Nasacort, deje de usar el pulverizador hasta que la infección haya sido tratada.
- si usted ha sido sometido recientemente a una operación en la nariz, o ha sufrido heridas o úlceras en la nariz.
- si usted está en proceso de transferencia de esteroides inyectables o en comprimidos a Nasacort suspensión para pulverización.
- si usted ha tenido glaucoma o cataratas.
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Si no está seguro de que lo descrito anteriormente sea aplicable a usted, consulte con su médico o farmacéutico antes de utilizar este medicamento.
Niños (menores de 2 años)
Este medicamento no está recomendado para el uso en niños menores de 2 años.
Cirugía o situaciones de estrés
Es posible que su médico le aconseje tomar una dosis de este medicamento mayor de lo habitual por razones médicas. En caso de un aumento de dosis, comuníquele a su médico si va a ser operado o no se encuentra bien. Esto es debido a que dosis más altas de las habituales de este medicamento pueden reducir la capacidad de su cuerpo para curarse o para superar el estrés. Si esto sucede, es posible que su médico decida que necesita un tratamiento adicional con otro medicamento para ayudarle.
Uso de Nasacort con otros medicamentos
Informe a su médico si está tomando o ha tomado recientemente otros medicamentos, incluso los adquiridos sin receta.
Algunos medicamentos pueden aumentar los efectos de Nasacort, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
No se conocen o son insignificantes los efectos de Nasacort sobre la capacidad para conducir o utilizar máquinas.
Nasacort contiene cloruro de benzalconio
Este medicamento contiene 15 microgramos de cloruro de benzalconio en cada pulverización.
El cloruro de benzalconio puede causar irritación o inflamación dentro de la nariz, especialmente cuando se usa durante un tratamiento a largo plazo.
Efectos sistémicos
Cuando los corticoesteroides, incluido el acetónido de triamcinolona, se utilizan durante largos periodos de tiempo o en dosis altas durante largos periodos de tiempo, pueden producirse efectos adversos debido a que el fármaco se absorbe en el organismo (p. ej., la producción normal de hormonas esteroideas puede verse afectada). Estos efectos adversos son mucho menos probables con los pulverizadores nasales con corticoesteroides que con los corticoesteroides tomados por vía oral (p. ej., comprimidos). Hable con su médico si experimenta algún efecto adverso.
3. Cómo usar Nasacort
NASACORT solo se debe usar por vía nasal
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico. Este medicamento sólo es eficaz si se utiliza de forma regular. Puede tardar de 3 a 4 días antes de apreciar una mejora de sus síntomas.
Cuánto Nasacort debe utilizar
Adultos y niños (mayores de 12 años)
- La dosis habitual de inicio es 2 pulverizaciones en cada fosa nasal por día.
- Una vez que los síntomas de alergia están controlados, se puede reducir la dosis a 1 pulverización en cada fosa nasal por día.
Niños (de 6 a 12 años)
- La dosis habitual es 1 pulverización en cada fosa nasal por día.
- Si los síntomas no desaparecen, es posible aumentar la dosis a 2 pulverizaciones en cada fosa nasal por día.
- Después se puede reducir la dosis a 1 pulverización en cada fosa nasal por día.
Niños (de 2 a 5 años)
- La dosis habitual es 1 pulverización en cada fosa nasal por día.
No use Nasacort durante más de 3 meses en niños menores de 12 años.
Un adulto debe ayudar a un niño pequeño a usar este medicamento.
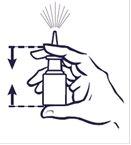
Cómo utilizar el pulverizador
Antes de usar su pulverizador nasal, debe sonarse la nariz con cuidado para despejar sus fosas nasales.
|
|
|
|
|
posición correcta posición incorrecta |
| |
| |
|
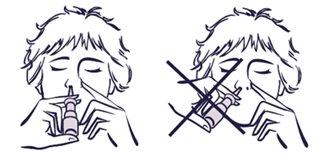
Si no ha utilizado el pulverizador durante más de dos semanas:
- Es necesario realizar el cebado de nuevo, para rellenar la boquilla con pulverizado.
- Debe dirigir la boquilla lejos de usted mientras esté realizando esta operación.
- Para el cebado, deberá hacer una pulverización al aire, antes de volver a utilizarlo.
- Agite siempre el frasco suavemente antes de su uso.
Limpieza del pulverizador
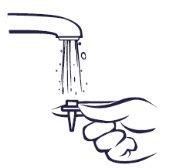
Si el pulverizador no funciona, es posible que la boquilla esté bloqueada. Nuncaintente desbloquearlo o hacer más grande el orificio del pulverizador con un alfiler o un objeto punzante. Esto podría hacer que el pulverizador dejara de funcionar.
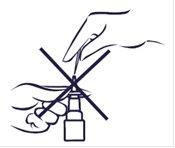
Deberá limpiar el pulverizador nasal al menos una vez a la semana. Se puede limpiar con más frecuencia si se bloquea.
Instrucciones para limpiar el pulverizador:
| |
Si usa más Nasacort del que debe
Es importante que tome su dosis tal y como se le ha indicado en la receta médica o como le ha aconsejado su médico.
Sólo debe usar este medicamento tal y como su médico le haya recomendado; sus síntomas podrían empeorar si lo utilizara más o menos de lo que se le ha indicado.
Es poco probable que se produzca una sobredosis, no obstante, si ha ingerido todo el contenido del envase de una sola vez por vía oral, podría experimentar molestias en el estómago o en el intestino. Hable con un médico si ha usado más Nasacort del que debe.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Nasacort
Si ha olvidado usar Nasacort, utilícelo tan pronto como pueda. No use una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con Nasacort
Si deja de usar este medicamento, sus síntomas podrían reaparecer en pocos días.
Si experimenta algún efecto adverso (síntomas de abstinencia) después de dejar de utilizar este medicamento repentinamente después de un uso prolongado, hable con su médico. Consulte la sección 4 “Posibles efectos adversos” para obtener más información.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Deje de utilizar Nasacort y acuda inmediatamente a un médico o un hospital si:
- presenta una reacción alérgica frente a Nasacort. Los síntomas (de frecuencia no conocida) pueden incluir: una erupción (urticaria), picor, problemas para tragar o para respirar, hinchazón de los labios, cara, garganta o lengua.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Goteo nasal, dolor de cabeza, garganta irritada y/o tos
- Sangrado de nariz
- Inflamación/irritación de las vías respiratorias (bronquitis)
- Acidez o indigestión
- Síntomas gripales (fiebre, dolor muscular, debilidad y/o fatiga)
- Problemas en los dientes
Otros efectos adversos(frecuencia no conocida: no puede estimarse a partir de los datos disponibles)
- Irritación y sequedad en el interior de su nariz
- Congestión o taponamiento de los senos nasales
- Estornudos
- Cambios en el gusto y el olor de las cosas
- Sensación de malestar (náuseas)
- Problemas de sueño, sensación de mareo o cansancio
- Respiración acortada (disnea)
- Descenso de los niveles de cortisol en sangre (valores analíticos)
- Vista nublada (cataratas), presión alta en el interior del ojo (glaucoma)
- Visión borrosa.
- La interrupción repentina de los corticosteroides después de un uso prolongado puede provocar síndrome de abstinencia de esteroides. Los síntomas de abstinencia como dolor en las articulaciones, dolor muscular, temblores, pérdida de peso, ansiedad, goteo y sangrado nasal se pueden observar con la triamcinolona intranasal. Sin embargo, estos síntomas son extremadamente raros en el caso de los pulverizadores nasales con corticoesteroides, y es mucho menos probable que ocurran con los pulverizadores nasales con corticoesteroides que con los corticoesteroides tomados por vía oral (p. ej., comprimidos).
En algunas personas, Nasacort puede dañar el interior de la nariz en su parte media (conocida como “septum nasal”). Consulte a su médico o farmacéutico cualquier duda que tenga sobre esto.
Otros efectos adversos en niños
Si su hijo ha estado utilizando este medicamento, éste puede afectar al crecimiento de su hijo. Esto significa que su médico necesitará comprobar la altura de su hijo de forma periódica ypor tanto su médico podría reducir la dosis. Además, su médico podría considerar recomendarle a un especialista pediátrico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Nasacort
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en el frasco después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
- No conservar a temperatura superior a 25ºC.
- Después de abrir Nasacort por primera vez, el formato de 30 pulverizaciones debe utilizarse en 1 mes y en 2 meses en el caso del formato de 120 pulverizaciones.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Nasacort
El principio activo es acetónido de triamcinolona. Una aplicación contiene 55 microgramos de acetónido de triamcinolona.
Los demás componentes son:
- celulosa microcristalina y carmelosa sódica (celulosa dispersable)
- polisorbato 80
- agua purificada
- glucosa anhidra
- cloruro de benzalconio (50% solución p/v),
- edetato disódico
- ácido clorhídrico o hidróxido sódico (para ajustar el pH)
Aspecto del producto y contenido del envase
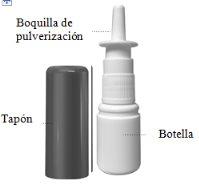
Nasacort es una suspensión para pulverización nasal. Se presenta en un frasco de plástico blanco con una bomba para suministrar la pulverización a través de una boquilla. El frasco presenta un tapón protector para que la boquilla se mantenga limpia y para evitar pulsaciones accidentales.
Este frasco único contiene al menos 120 pulverizaciones (16,5 g de suspensión con 9,075 mg de acetónido de triamcinolona) o al menos 30 pulverizaciones (6,5 g de suspensión con 3,575 mg de acetónido de triamcinolona).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Opella Healthcare Spain, S.L.
C/ Rosselló i Porcel, 21
08016 Barcelona
España
Grupo Sanofi
Responsable de la fabricación:
Bespak HC LimitedLondon Road, Holmes Chapel
Cheshire, CW4 8BE
Reino Unido
o
Sanofi Winthrop Industrie
30-36, avenue Gustave Eiffel
37100 Tours
Francia
o
Sanofi-Aventis Deutschland GmbH
65926 Frankfurt am Main
Alemania
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con el siguiente nombre: Nasacort
Austria: Nasacort Nasenspray
Bélgica: Nasacort
Bulgaria: Nasacort
República Checa: Triamcinolone sanofi 55 mikrogramu/dávka
Dinamarca: Nasacort
Estonia: Nasacort
Finlandia: Nasacort
Alemania: Nasacort 55 Mikrogramm/Dosis
Grecia: Nasacort
Hungría: Nasacort
Irlanda: Nasacort nasal spray
Italia: Nasacort
Letonia: Nasacort
Lituania: Triamcinolone acetonide Sanofi 55μg/dose nasal spray, suspension
Luxemburgo: Nasacort
Holanda Nasacort
Polonia: Nasacort
Portugal: Nasacort
Rumania: Nasacort 55 micrograme/doza spray nazal, suspensie
Eslovaquia: Nasacort 55 mikrogramov/dávka
Eslovenia: Nasacort
España: Nasacort 55 microgramos/dosis, suspensión para pulverización nasal
Suecia: Nasacort
Reino Unido: Nasacort nasal spray
Fecha de la última revisión de este prospecto: Agosto 2022
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia15.55 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NASACORT 55 microgramos/dosis, SUSPENSION PARA PULVERIZACION NASALForma farmacéutica: PRODUCTO USO NASAL, 27,5 µgPrincipio activo: fluticasona furoatoFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: PRODUCTO USO NASAL, 27,5 MICROGRAMOS/PULVERIZACIÓNPrincipio activo: fluticasona furoatoFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 137 microgramos/50 microgramos/aplicaciónPrincipio activo: fluticasone, combinationsFabricante: Laboratorios Cinfa S.A.Requiere receta
Médicos online para NASACORT 55 microgramos/dosis, SUSPENSION PARA PULVERIZACION NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NASACORT 55 microgramos/dosis, SUSPENSION PARA PULVERIZACION NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes