
IOPIMAX 5 mg/ml EYE DROPS SOLUTION


How to use IOPIMAX 5 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
IOPIMAX 5mg/ml eye drops solution
Apraclonidine hydrochloride
Read all of this leaflet carefullybefore you start using this medicine because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is IOPIMAX and what is it used for
- What you need to know before you use IOPIMAX
- How to use IOPIMAX
- Possible side effects
- Storing IOPIMAX
- Contents of the pack and further information
1. What is IOPIMAX and what is it used for
IOPIMAX5 mg/ml eye drops solution contains apraclonidine (as hydrochloride) as the active substance.
IOPIMAXbelongs to a group of medicines called alpha-agonists.
It is used for the treatment ofchronic glaucoma in people who are already taking other medicines to treat this disease.
In this group of people, IOPIMAX helps to reduce the pressure of fluids in the eye and to delay surgical intervention to relieve eye pressure.
2. What you need to know before you use IOPIMAX
Do not use IOPIMAX
- if you have a history of severe or unstable heart diseaseor circulation problems.
- if you are allergicto apraclonidine or any of the other ingredients of this medicine (listed in section 6).
- if you are currently taking antidepressants that belong to the class of monoamine oxidase inhibitors or tricyclic antidepressants.
- if you are currently taking medicines of the class of sympathomimetics that are taken orally or by injection.
- in CHILDREN.
If you are unsure, consult your doctor.
Warnings and precautions
Be particularly careful:
- Only use this medicine in your eye(s).
- IOPIMAX may not continue to control the pressure in your eyes after you have used it for a period of time. Your doctor will examine your eyes frequently while you are using IOPIMAX to check that the eye drops are still working.
- Since IOPIMAX reduces the pressure in the eye(s), you should have your eye pressure checked regularly to ensure that the pressure in the eye(s) remains under control.
If you have a history ofor are being treated forany of the following diseases:
- Any heart disease(including angina, infarction, or heart failure)
- High blood pressureor other circulation problems(including embolisms, Raynaud's disease, and fainting)
- Kidney or liver problems
- Depression
- Parkinson's disease
- Diabetes or low blood sugar levels. IOPIMAX may hide the signs and symptoms of a sudden drop in blood sugar, such as rapid heartbeat or tremors
- If you are scheduled for surgery
If you think you are affected by any of the above cases, you may still use IOPIMAX, but consult your doctor first.
Using IOPIMAX with food, drinks, and alcohol
Do not consume alcoholduring treatment with IOPIMAX because it may enhance its effects.
Other medicines and IOPIMAX
Tell your doctor or pharmacist if you are using or have recently used or might use any other medicines.
Do not use IOPIMAXif you are taking antidepressants that belong to the class of monoamine oxidase inhibitors or tricyclic antidepressants. Also, do not use IOPIMAX if you are taking medicines of the class of sympathomimetics that are taken orally or by injection.
IOPIMAX may increase the effectsof other medicines used to treat depression, asthma, high blood pressure, heart medicines that contain digoxin or digitoxin, various types of mental illnesses, and Parkinson's disease. It may interact with some pain-relieving medicines, sedatives, anesthetics, tricyclic antidepressants, cough and cold remedies, glaucoma medications such as timolol or brimonidine, and eye drops used to whiten the eye.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use this medicine during pregnancy or breastfeeding.
Driving and using machines
This type of medication may cause you to feel drowsy and dizzy. If so, do not driveor use machines.
IOPIMAX contains benzalkonium chloride
This medicine contains 0.1 mg of benzalkonium chloride per ml, equivalent to 0.01%.
IOPIMAX contains a preservative (benzalkonium chloride) that can be absorbed by soft contact lenses and may alter the color of the contact lenses. You should remove your contact lenses before using this medicine and wait 15 minutes before putting them back in. Benzalkonium chloride can cause eye irritation, especially if you have dry eyes or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medicine.
3. How to use IOPIMAX
Recommended dose
The recommended dose is 1 drop in the eye(s) 3 times a day.
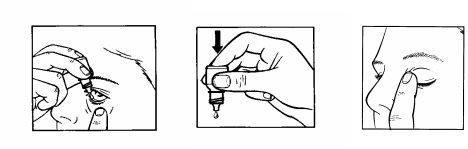
1 2 3
Remove the collarthat surrounds the cap when you open the bottle for the first time.
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
How to use it
- Wash your hands before starting.
- Remove the cap.
- Hold the bottle upside down between your thumb and fingers.
- Tilt your head back.
- Pull your lower eyelid down with one finger until a pocket is formed between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, or surrounding areas with the dropper. The drops could become infected.
- Gently squeeze the bottle to release one drop at a time (figure 2).
- Do not squeeze the bottle; gentle pressure is enough.
- After using IOPIMAX, press the edge of your eye, next to your nose, with your finger. This helps prevent IOPIMAX from entering the rest of your body (figure 3).
- If you are applying drops to both eyes, repeat the above steps for the other eye. Close the bottle tightly after use.
- If a drop falls outside the eye, try again.
- In case of ingestion of IOPIMAX, consult your doctor immediately.
If you forget to use IOPIMAX
Continue with the next dose that was scheduled. However, if it is almost time for the next dose, do not apply the missed dose and continue with the next dose of your regular schedule.
Do not apply a double dose to make up for the missed dose.
If you use more IOPIMAX than you should
You can rinse your eyes with warm water. Do not apply more drops until it is time for your next dose. In case of accidental ingestion, the symptoms of overdose may include: low blood pressure, drowsiness, decreased heart rate, shallow breathing (reduced rate and depth of breathing that causes increased carbon dioxide), and convulsions.
If you are using other eye medicines, wait at least 5 minutes between the administration of this eye drop and the other eye medicines. Eye ointments should be applied last.
If you have any further questionsabout the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
IOPIMAX may cause allergic reactions. If you experienceone or more of the following symptoms in the eye(s): redness, itching, watery eyes, abnormal sensation, or swelling of the eye or eyelid, consult your doctor immediately.
If your vision worsensafter using IOPIMAX, stop using itand consult your doctor immediately.
You may experience some or all of the following reactions in your eye(s):
Very common (may affect more than 1 in 10 people):increased redness, itching, inflammation.
Common (may affect up to 1 in 10 people):discomfort, increased tear production, eyelid swelling, feeling of grit in the eye, dry eye, crusts on the eyelids.
Uncommon (may affect up to 1 in 100 people):swelling under the eyelids, swelling of the eye, abnormal vision, pain, inflammation, and irritation of the eye or eyelids, damage to the corneal surface (the front part of your eye), sensitivity to light, redness of the eyelid, increased or decreased eyelid size, increased pupil size, reduced vision, blurred vision, drooping eyelid, discharge or whitening of the eye.
You may also experience effects in other parts of your body, including:
Common (may affect up to 1 in 10 people):dry mouth, inflammation inside the nose, dermatitis, dry nose, weakness, headache, unusual taste.
Uncommon (may affect up to 1 in 100 people):chest pain, swelling of hands, feet, or limbs, irregular heartbeat, constipation, nausea, feeling of tiredness, throat inflammation, runny nose, muscle pain, abnormal coordination, drowsiness, dizziness, tingling sensation, feeling of nervousness, depression, difficulty sleeping, reduced air exhaled or difficulty breathing, unusual smell sensation, facial swelling, irritability, dilation of blood vessels.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing IOPIMAX
- Keep this medicine out of the sight and reach of children.
- Do not store above 25°C. Do not refrigerate or freeze.
- Keep the bottle tightly closed and store in the outer packaging.
- Do not use this medicine after the expiry date which is stated on the bottle and carton after “EXP”. The expiry date is the last day of the month stated.
- To avoid infections, you must discard the bottle 4weeks after first opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of IOPIMAX
- The active substance is apraclonidine 5 mg/ml (as hydrochloride).
- The other ingredients are sodium chloride, sodium acetate (trihydrate), benzalkonium chloride, hydrochloric acid, and/or sodium hydroxide (for pH adjustment) and purified water.
Appearance and packaging
IOPIMAX is a clear, colorless or slightly yellowish solution, presented in a carton containing a 5 ml or 10 ml plastic bottle with a screw cap.
Marketing authorization holder
Essential Pharma Limited,
Vision Exchange Building
Triq it-Territorjals, Zone 1,
Central Business District,
Birkirkara, CBD 1070,
Malta
Manufacturer
Essential Pharma Limited,
Vision Exchange Building
Triq it-Territorjals, Zone 1,
Central Business District,
Birkirkara, CBD 1070,
Malta
Date of last revision of this leaflet:February 2023.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://aemps.gob.es/
- Country of registration
- Average pharmacy price11.1 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IOPIMAX 5 mg/ml EYE DROPS SOLUTIONDosage form: EYE DROP, 10 mg/ml of apraclonidine (as hydrochloride)Active substance: apraclonidineManufacturer: Essential Pharma LimitedPrescription requiredDosage form: EYE DROP, 2 mg/mlActive substance: brimonidineManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 2 mg/ml of brimonidine tartrateActive substance: brimonidineManufacturer: Abbvie Spain, S.L.U.Prescription required
Online doctors for IOPIMAX 5 mg/ml EYE DROPS SOLUTION
Discuss questions about IOPIMAX 5 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















