
IMRALDI 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA


Cómo usar IMRALDI 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Imraldi 40 mg solución inyectable en pluma precargada
adalimumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Su médico también le entregará una tarjeta de información para el paciente, que contiene información de seguridad importante que necesita conocer antes y durante el tratamiento con Imraldi. Conserve esta tarjeta de información para el paciente durante su tratamiento y los 4 meses posteriores a su última inyección (o de su hijo) de Imraldi.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Imraldi y para qué se utiliza
- Qué necesita saber antes de empezar a usar Imraldi
- Cómo usar Imraldi
- Posibles efectos adversos
- Conservación de Imraldi
- Contenido del envase e información adicional
- Instrucciones de uso
1. Qué es Imraldi y para qué se utiliza
Imraldi contiene como sustancia activa adalimumab, un medicamento que atúa sobre el sistema inmunitario (de defensa) de su organismo.
Imraldi está indicado en el tratamiento de:
- artritis reumatoide,
- artritis idiopática juvenil poliarticular,
- artritis asociada a entesitis,
- espondilitis anquilosante,
- espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante,
- artritis psoriásica,
- psoriasis,
- hidradenitis supurativa,
- enfermedad de Crohn,
- colitis ulcerosa y
- uveítis no infecciosa.
El principio activo de Imraldi, adalimumab, es un anticuerpo monoclonal. Los anticuerpos monoclonales son proteínas que se unen a una diana específica.
La diana de adalimumab es una proteína (llamada factor de necrosis tumoral (TNFa), cuyos niveles aumentan en las enfermedades inflamatorias indicadas anteriormente. Al unirse a la TNFa, Imraldi reduce el proceso de la inflamación en estas enfermedades.
Artritis reumatoide
La artritis reumatoide es una enfermedad inflamatoria de las articulaciones.
Imraldi se utiliza para tratar la artritis reumatoide en adultos. Si usted padece artritis reumatoide activa moderada a grave, puede que se le administren antes otros medicamentos modificadores de la enfermedad tales como metotrexato. Si el efecto de estos medicamentos no es suficientemente bueno, se le administrará Imraldi para tratar su artritis reumatoide.
Imraldi también puede usarse en el tratamiento de la artritis reumatoide grave, activa y progresiva sin tratamiento previo con metotrexato.
Imraldi puede reducir el daño de los cartílagos y huesos de las articulaciones producido por la enfermedad y que mejora el rendimiento físico.
Habitualmente Imraldi se usa junto con metotrexato. Si su médico considera que el metotrexato no es apropiado, Imraldi puede administrarse solo.
Artritis idiopática juvenil poliarticular y artritis asociada a entesitis
La artritis idiopática juvenil poliarticular y la artritis asociada a entesitis son enfermedades inflamatorias de las articulacions que suelen aparecer en la infancia.
Imraldi se utiliza para tratar la artritis idiopática juvenil poliarticular en niños y adolescentes de 2 a 17 años de edad y la artritis asociada a entesitis en niños entre 6 y 17 años. Los pacientes pueden haber recibido primero otros fármacos modificadores de la enfermedad, como metotrexato. Si el efecto de estos medicamentos no es suficientemente bueno, a los pacientes se les administrará Imraldi para tratar su artritis idiopática poliarticular o artritis asociada a entesitis.
Espondilitis anquilosante y espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante
La espondilitis anquilosante y la espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante son enfermedades inflamatorias que afectan a la columna vertebral.
Imraldi se utiliza para tratar la espondilitis anquilosante y la espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante en adultos. Si tiene espondilitis anquilosante o espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante, será tratado primero con otros medicamentos y si el efecto de estos medicamentos no es suficientemente bueno, recibirá Imraldi para reducir los signos y síntomas de su enfermedad.
Artritis psoriásica
La artritis psoriásica es una enfermedad inflamatoria de las articulaciones asociada con la psoriasis.
Imraldi se utiliza para tratar la artritis psoriásica en adultos. Imraldi puede reducir el daño articular que produce la enfermedad en el cartílago y en el hueso y mejora el rendimiento físico.
Psoriasis en placas en adultos y niños
La psoriasis en placas es una enfermedad inflamatoria de la piel que causa áreas enrojecidas, escamosas, con costras y cubiertas por escamas plateadas. La psoriasis en placas también puede afectar las uñas, provocando que se deterioren, se engrosen y se levanten del lecho de la uña, lo cual puede ser doloroso. Se cree que la psoriasis está causada por un defecto en el sistema inmune del cuerpo que lleva a un incremento en la producción de células de la piel.
Imraldi se utiliza para tratar la psoriasis en placas de moderada a grave en adultos. Imraldi también se utiliza para tratar la psoriasis en placas grave en niños y adolescentes con un peso igual o superior a 30 kg que no hayan respondido o no sean buenos candidatos para terapia tópica y fototerapias.
Hidradenitis supurativa en adultos y adolescentes
La hidradenitis supurativa (a veces denominada acné inverso) es una enfermedad inflamatoria de la piel de larga duración y a menudo dolorosa. Los síntomas pueden incluir nódulos sensibles (bultos) y abscesos (forúnculos) que pueden secretar pus. Normalmente afecta a áreas específicas de la piel, como debajo del pecho, de las axilas, zona interior de los muslos, ingle y nalgas. También puede haber cicatrices en las áreas afectadas.
Imraldi se utiliza para tratar la hidradenitis supurativa en adultos y adolescentes a partir de los 12 años de edad. Imraldi puede reducir el número de nódulos y abscesos, y el dolor que normalmente va asociado a su enfermedad. Puede haber recibido otros medicamentos previamente. Si el efecto de estos medicamentos no es suficientemente bueno, recibirá Imraldi.
Enfermedad de Crohn en adultos y niños
La enfermedad de Crohn es una enfermedad inflamatoria del tubo digestivo.
Imraldi se utiliza para tratar la enfermedad de Crohn en adultos y niños de edades comprendidas entre los 6 y los 17 años. Si padece enfermedad de Crohn será tratado primero con otros medicamentos. Si el efecto no es suficientemente bueno, recibirá Imraldi para reducir los signos y síntomas de la enfermedad de Crohn.
Colitis ulcerosa en adultos y niños
La colitis ulcerosa es una enfermedad inflamatoria del intestino grueso.
Imraldi se utiliza para tratar la colitis ulcerosa de moderada a grave en adultos y niños de edades comprendidas entre los 6 y los 17 años. Si usted sufre colitis ulcerosa puede que primero le receten otros medicamentos. Si el efecto no es suficientemente bueno, le recetarán Imraldi para reducir los signos y los síntomas de su enfermedad.
Uveítis no infecciosa en adultos y niños
La uveítis no infecciosa es una enfermedad inflamatoria que afecta ciertas partes del ojo. Imraldi se utiliza para tratar
- adultos con uveítis no infecciosa con inflamación que afecta la zona posterior del ojo.
- niños desde los 2 años de edad con uveítis crónica no infecciosa con inflamación que afecta a la parte frontal del ojo.
Esta inflamación puede conducir a una disminución de la visión y/o la presencia de motas en el ojo (puntos negros o líneas delgadas que se mueven a lo largo del campo de visión). Imraldi actúa reduciendo esta inflamación.
2. Qué necesita saber antes de empezar a usar Imraldi
No use Imraldi
- Si es alérgico a adalimumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si padece una infección grave, incluyendo tuberculosis (ver “Advertencias y precauciones”). En caso de tener síntomas de cualquier infección, por ejemplo: fiebre, heridas, cansancio, problemas dentales, es importante que informe a su médico.
- Si padece insuficiencia cardiaca moderada o grave. Es importante que le diga a su médico si ha tenido o tiene algún problema cardiaco serio (ver “Advertencias y precauciones”).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Imraldi.
Reacción alérgica
- Si tiene una reacción alérgicacon síntomas como opresión en el pecho, dificultad para respirar, mareo, hinchazón o sarpullido, interrumpa la administración de Imraldi y póngase en contacto con su médico inmediatamente ya que, en casos raros, estas reacciones pueden poner en peligro la vida.
Infección
- Si padece cualquier infección, incluyendo las crónicas o las localizadas (por ejemplo: una úlcera en la pierna), consulte a su médico antes de comenzar el tratamiento con Imraldi. Si no está seguro, póngase en contacto con su médico.
- Con el tratamiento con Imraldi podría contraer infecciones con más facilidad. Este riesgo puede ser mayor si disminuye la actividad de sus pulmones. Estas infecciones pueden ser graves e incluyen tuberculosis, infecciones causadas por virus, hongos, parásitos o bacterias, otras infecciones oportunistas (infecciones raras asociadas a un sistema inmunitario debilitado) y sepsis (envenenamiento de la sangre). En casos raros, estas infecciones pueden poner en peligro su vida. Por esta razón es importante que en el caso de que tenga síntomas como fiebre, heridas, cansancio o problemas dentales, se lo diga a su médico. Su médico le puede recomendar que interrumpa temporalmente el tratamiento con Imraldi.
Tuberculosis
- Dado que se han descrito casos de tuberculosis en pacientes en tratamiento con Imraldi, su médico le examinará en busca de signos o síntomas de tuberculosis antes de comenzar su tratamiento con Imraldi. Esto incluirá la realización de una evaluación médica minuciosa, incluyendo su historia médica y las pruebas de diagnóstico (por ejemplo radiografía de tórax y la prueba de la tuberculina). La realización y resultados de estas pruebas debe anotarse en su tarjeta de información para el paciente. Es muy importante que informe a su médico en caso de haber padecido tuberculosis o haber estado en contacto con un paciente de tuberculosis. Se puede desarrollar tuberculosis durante el tratamiento incluso si usted ha recibido tratamiento preventivo para la tuberculosis. Si apareciesen síntomas de tuberculosis (tos persistente, pérdida de peso, malestar general, febrícula) o de cualquier otra infección durante o una vez finalizado el tratamiento, póngase en contacto inmediatamente con su médico.
Infección por viaje/recurrente
- Informe a su médico si ha residido o viajado por regiones en las que infecciones fúngicas como histoplasmosis, coccidiomicosis o blastomicosis son endémicas.
- Informe a su médico si tiene antecedentes de infecciones recurrentes u otras patologías o factores que aumenten el riesgo de infecciones.
Virus de la hepatitis B
- Informe a su médico si es usted portador del virus de la hepatitis B (VHB), si ha tenido una infección activa con VHB o si piensa que podría correr riesgo de contraer el VHB. Su médico le debe realizar un análisis para el VHB. Imraldi puede reactivar la infección con VHB en personas portadoras de este virus. En casos raros, especialmente si está tomando otros medicamentos que suprimen el sistema inmune, la reactivación de la infección con VHB puede poner en peligro su vida.
Paciente mayor de 65 años
- Si tiene más de 65 años puede ser más susceptible de padecer infecciones mientras está en tratamiento con Imraldi. Tanto usted como su médico deben prestar atención especial a la aparición de signos de infección mientras esté siendo tratado con Imraldi. Es importante informar a su médico si tiene síntomas de infecciones, como fiebre, heridas, sensación de cansancio o problemas dentales.
Intervención quirúrgica o dental
- Si le van a realizar una intervención quirúrgica o dental, informe a su médico de que está tomando Imraldi. Su médico le puede recomendar que suspenda temporalmente el tratamiento con Imraldi.
Enfermedad demielinizante
- Si padece o desarrolla una enfermedad desmielinizante(una enfermedad que afecta a la capa aislante que rodea los nervios, como la esclerosis múltiple), su médico decidirá si debe ser tratado o continuar en tratamiento con Imraldi. Informe inmediatamente a su médico si siente síntomas tales como cambios en la visión, debilidad en brazos o piernas o entumecimiento u hormigueo en cualquier parte del cuerpo.
Vacunas
- Ciertas vacunasconienen formas vivas, aunque debilitadas, de bacterias o virus causantes de enfermedades y estas vacunas no deben administrarse durante el tratamiento con Imraldi. Consulte con su médico antes de la administración de cualquier tipo de vacuna. Si es posible, administrar a los niños todas las vacunas programadas para su edad antes de iniciar el tratamiento con Imraldi. Si recibe Imraldi mientras está embarazada, su hijo puede tener un riesgo mayor de sufrir infecciones durante unos 5 meses después de la última dosis que haya recibido de Imraldi durante su embarazo. Es importante que informe al médico de su hijo y a otros profesionales sanitarios sobre su uso de Imraldi durante el embarazo, para que ellos puedan decidir si su hijo debe recibir alguna vacuna.
Insuficiencia cardíaca
- Si padece insuficiencia cardíaca levey está en tratamiento con Imraldi, su médico debe hacerle un seguimiento continuo de su insuficiencia cardíaca. Es importante que informe a su médico si ha padecido o padece problemas serios de corazón. En caso de que aparezcan nuevos síntomas de insuficiencia cardíaca o empeoren los actuales (por ejemplo: dificultad al respirar, o hinchazón de los pies), debe ponerse en contacto con su médico inmediatamente. Su médico decidirá si debe seguir tomando Imraldi.
Fiebre, hematomas, sangrado o aspecto pálido
- En algunos pacientes, el organismo puede ser incapaz de producir un número suficiente del tipo de células sanguíneas para luchar contra las infecciones (glóbulos blancos) o de las que contribuyen a parar las hemorragias (plaquetas). Si tiene fiebrepersistente, o sufre cardenaleso sangramuy fácilmente o está muy pálido, consulte inmediatamente a su médico. Su médico puede decidir la interrupción del tratamiento.
Cáncer
- En muy raras ocasiones se han dado casos de ciertos tipos de cánceren niños y adultos tratados con Imraldi u otros agentes que bloquean el TNFa. Las personas con artritis reumatoide de grados más graves y que padezcan la enfermedad desde hace mucho tiempo pueden tener un riesgo mayor que la media de desarrollar un linfoma(un cáncer que afecta al sistema linfático), y leucemia (un cáncer que afecta a la sangre y a la médula ósea). Si está en tratamiento con Imraldi el riesgo de padecer linfoma, leucemia y otros tipos de cáncer puede incrementarse. Se ha observado, en raras ocasiones, un tipo de linfoma específico y grave en pacientes en tratamiento con Imraldi. Algunos de estos pacientes recibían tratamiento también con los medicamentos azatioprina o mercaptopurina. Informe a su médico si está tomando azatioprina o mercaptopurina con Imraldi.
- Además se han observado casos de cáncer de piel (tipo no melanoma)en pacientes que usan Imraldi. Avise a su médico si durante o después del tratamiento aparecen nuevas zonas de piel dañadas o si las marcas o las zonas dañadas cambian de apariencia.
- Se han registrado cánceres, diferentes del linfoma, en pacientes con una determinada enfermedad pulmonar, denominada enfermedad pulmonar obstructiva crónica (EPOC), tratados con otro agente bloqueante del TNFa. Si tiene EPOC, o fuma mucho, debe consultar a su médico si el tratamiento con un bloqueante del TNFaes adecuado en su caso.
Síndrome similar al lupus
- En raras ocasiones el tratamiento con Imraldi podría dar lugar a un síndrome similar al lupus. Contacte con su médico si tiene síntomas como erupción persistente sin explicación, fiebre, dolor de las articulaciones o cansancio.
Niños y adolescentes
- No administre Imraldi a niños menores de 2 años con artritis idiopática juvenil poliarticular.
- No use la pluma precargada de 40 mg si se recomiendan dosis diferentes a 40 mg.
Otros medicamentos e Imraldi
Informe a su médico o farmacéutico si está tomando, ha tomado utilizado recientemente o pudiera tener que tomar cualquier otro medicamento.
Imraldi se puede tomar junto con metotrexato o con ciertos medicamentos antirreumáticos modificadores de la enfermedad (sulfasalazina, hidroxicloroquina, leflunomida y preparaciones inyectables a base de sales de oro), esteroides o medicamentos para el dolor, incluidos los antiinflamatorios no esteroideos (AINEs).
No debe utilizar Imraldi junto con medicamentos cuyos principios activos sean anakinra o abatacept debido a un incremento del riesgo de infecciones graves. Si tiene alguna duda, consulte a su médico.
Embarazo y lactancia
- Debe considerar el uso de métodos anticonceptivos adecuados para evitar quedarse embarazada y continuar con su uso durante al menos 5 meses después de la última inyección de Imraldi.
- Si está embarazada, cree que podría estar embarazada o tiene intención de tener un bebé, pida consejo a su médico antes de usar este medicamento.
- Imraldi debe usarse durante el embarazo solo si es necesario.
- Según un estudio en embarazo, no hubo un mayor riesgo de defectos congénitos cuando la madre había recibido tratamiento con adalimumab durante el embarazo comparado con las madres con la misma enfermedad que no recibieron tratamiento con adalimumab.
- Imraldi puede usarse durante la lactancia.
- Si utiliza Imraldi mientras está embarazada, su hijo puede tener un riesgo más alto de contraer una infección.
- Es importante que informe al pediatra y a otros profesionales sanitarios sobre el uso de Imraldi durante el embarazo antes de que el bebé reciba ninguna vacuna. Para más información sobre vacunas ver la sección “Advertencias y precauciones”
Conducción y uso de máquinas
La influencia de Imraldi sobre la capacidad para conducir, montar en bicicleta o utilizar máquinas es pequeña. Puede producirse sensación de que la habitación da vueltas (vértigo) y alteraciones de la visión después de tomar Imraldi.
Imraldi contiene sodio y sorbitol
Sorbitol
Este medicamento contiene 20 mg de sorbitol en cada pluma precargada. Si su médico le ha informado de que usted padece intolerancia a ciertos azúcares, póngase en contacto con él antes de tomar este medicamento.
Sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis de 0,8 ml; esto es, esencialmente “exento de sodio”.
3. Cómo usar Imraldi
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Adultos con artritis reumatoide, artritis psoriásica, espondilitis anquilosante o espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante
Imraldi en jeringa precarga y en pluma precargada solo están disponibles en dosis de 40 mg. Por lo tanto, no es posible administrar Imraldi en jeringa precarga o pluma precargada a los pacientes pediátricos que requieren menos de una dosis completa de 40 mg. Cuando se requiera una dosis alternativa, deben utilizarse otras presentaciones que ofrezcan dicha opción.
Imraldi se inyecta bajo la piel (vía subcutánea). La dosis normal en adultos con artritis reumatoide, espondilitis anquilosante, espondiloartritis axial sin evidencia radiográfica de espondilitis anquilosante y para pacientes con artritis psoriásica es de 40 mg de adalimumab administrados en semanas alternas como dosis única.
En el caso de la artritis reumatoide, el tratamiento con metotrexato se mantiene durante el uso de Imraldi. Si su médico determina que el metotrexato es inapropiado, Imraldi puede administrarse solo.
Si usted padece artritis reumatoide y no recibe metotrexato durante su tratamiento con Imraldi, su médico puede decidir darle 40 mg de adalimumab cada semana u 80 mg cada dos semanas.
Niños, adolescentes y adultos con artritis idiopática juvenil poliarticular
Niños y adolescentes desde 2 años de edad con 10 kg de peso hasta 30 kg
La dosis recomendada de Imraldi es 20 mg en semanas alternas.
Niños, adolescentes y adultos desde 2 años de edad con peso de 30 kg o más
La dosis recomendada de Imraldi es 40 mg en semanas alternas.
Niños, adolescentes y adultos con artritis asociada a entesitis
Niños y adolescentes desde 6 años de edad con 15 kg de peso hasta 30 kg
La dosis recomendada de Imraldi es 20 mg en semanas alternas.
Niños, adolescentes y adultos desde 6 años de edad con peso de 30 kg o más
La dosis recomendada de Imraldi es 40 mg en semanas alternas.
Adultos con psoriasis
La posología normal en adultos con psoriasis consiste en una dosis inicial de 80 mg (como dos inyecciones de 40 mg en un día), seguida de 40 mg en semanas alternas comenzando una semana después de la dosis inicial. Debe continuar inyectándose Imraldi durante tanto tiempo como le haya indicado su médico. Si esa dosis no funciona lo suficientemente bien, su médico puede incrementar la dosis a 40 mg semanales u 80 mg cada dos semanas.
Niños y adolescentes con psoriasis en placas
Niños y adolescentes desde 4 a 17 años de edad con 15 kg de peso hasta 30 kg
La dosis recomendada de Imraldi es una dosis inicial de 20 mg seguida de 20 mg una semana después. A partir de entonces, la dosis habitual es de 20 mg en semanas alternas.
Niños y adolescentes desde 4 a 17 años de edad con peso de 30 kg o más
La dosis recomendada de Imraldi es una dosis inicial de 40 mg, seguida de 40 mg una semana después. A partir de entonces, la dosis habitual es de 40 mg en semanas alternas.
Adultos con hidradenitis supurativa
La pauta de dosificación habitual para la hidradenitis supurativa es de una dosis inicial de 160 mg (como cuatro inyecciones de 40 mg en un día o dos inyecciones de 40 mg al día durante dos días consecutivos) seguida por una dosis de 80 mg (como dos inyecciones de 40 mg en día) dos semanas después. Después de dos semanas más, continuar con una dosis de 40 mg semanales u 80 mg cada dos semanas, según se lo haya recetado su médico. Se recomienda que utilice a diario un líquido antiséptico en las zonas afectadas.
Adolescentes con hidradenitis supurativa a partir de 12 a 17 años de edad, con un peso de 30 kg o más
La dosis recomendada de Imraldi es una dosis inicial de 80 mg (como dos inyecciones de 40 mg en un día), seguida por 40 mg en semanas alternas a partir de una semana más tarde. Si tiene una respuesta inadecuada a Imraldi 40 mg en semanas alternas, su médico puede incrementar la dosis a 40 mg semanales 80 mg cada dos semanas.
Se recomienda que utilice a diario un líquido antiséptico en las zonas afectadas.
Adultos con enfermedad de Crohn
El régimen de dosificación habitual para la enfermedad de Crohn es de 80 mg (como dos inyecciones de 40 mg en un día) inicialmente, seguido de 40 mg en semanas alternas comenzando dos semanas después.
Si se requiere un efecto más rápido, su médico puede recetarle una dosis inicial de 160 mg (como cuatro inyecciones de 40 mg en un día o dos inyecciones de 40 mg por día durante dos días consecutivos), seguido de 80 mg (como dos inyecciones de 40 mg en un día) dos semanas después, y a partir de entonces 40 mg en semanas alternas. Si el efecto de esta dosis no es suficiente, su médico puede incrementar la dosis a 40 mg semanales u 80 mg cada dos semanas.
Niños y adolescentes con enfermedad de Crohn
Niños y adolescentes desde 6 a 17 años de edad con peso menor a 40 kg
El régimen de dosificación habitual es de 40 mg inicialmente seguido de 20 mg dos semanas después. Si se requiere una respuesta más rápida, su médico puede recetarle una dosis inicial de 80 mg (como dos inyecciones de 40 mg en un día) seguido de 40 mg dos semanas después.
A partir de entonces, la dosis habitual es de 20 mg en semanas alternas. Si esa dosis no funciona lo suficientemente bien, su médico puede incrementar la frecuencia de la dosis a 20 mg semanales.
Niños y adolescentes desde 6 a 17 años de edad con peso de 40 kg o más:
El régimen de dosificación habitual es de 80 mg (como dos inyecciones de 40 mg en un día) inicialmente seguido de 40 mg dos semanas más tarde. Si se requiere una respuesta más rápida, su médico puede recetarle una dosis inicial de 160 mg (como cuatro inyecciones de 40 mg en un día o dos inyecciones de 40 mg al día durante dos días consecutivos) seguido de 80 mg (como dos inyecciones de 40 mg en un día) dos semanas después.
A partir de entonces, la dosis habitual es de 40 mg en semanas alternas. Si el efecto de esta dosis no es suficiente, su médico puede incrementar la dosis a 40 mg semanales u 80 mg cada dos semanas.
Adultos con colitis ulcerosa
El régimen de dosificación habitual de Imraldi en adultos con colitis ulcerosa es de 160 mg inicialmente (la dosis se la puede administrar mediante cuatro inyecciones en un día o con dos inyecciones de 40 mg por día durante dos días seguidos) seguido de 80 mg (como dos inyecciones de 40 mg en un día) dos semanas más tarde, y a partir de entonces 40 mg en semanas alternas. Si el efecto de esta dosis no es suficiente, su médico puede incrementar la dosis a 40 mg semanales u 80 mg cada dos semanas.
Niños y adolescentes con colitis ulcerosa
Niños y adolescentes desde 6 años con un peso inferior a 40 kg
La dosis habitual de Imraldi es de 80 mg (como dos inyecciones de 40 mg en un día) inicialmente, seguida de una dosis de 40 mg (como una inyección de 40 mg) dos semanas después. A partir de entonces, la dosis habitual es de 40 mg en semanas alternas.
Los pacientes que cumplan 18 años mientras reciben tratamiento con 40 mg en semanas alternas deben continuar con su dosis prescrita.
Niños y adolescentes desde 6 años con un peso de 40 kg o superior
La dosis habitual de Imraldi es de 160 mg (como cuatro inyecciones de 40 mg en un día o dos inyecciones de 40 mg al día en dos días consecutivos) inicialmente, seguida de una dosis de 80 mg (como dos inyecciones de 40 mg en un día) dos semanas después. A partir de entonces, la dosis habitual es de 80 mg en semanas alternas.
Los pacientes que cumplan 18 años mientras reciben tratamiento con 80 mg en semanas alternas deben continuar con su dosis prescrita.
Adultos con uveítis no infecciosa
El régimen de dosificación habitual en adultos con uveítis no infecciosa es de 80 mg (como dos inyecciones en un día) inicialmente, seguido de 40 mg en semanas alternas empezando una semana después de la dosis inicial. Continúe inyectando Imraldi durante el tiempo que le haya indicado su médico.
En la uveítis no infecciosa se puede continuar con la administración de corticoesteroides o de los medicamentos que actúan sobre el sistema inmunitario durante el uso de Imraldi. Imraldi también se puede administrar solo.
Niños y adolescentes desde los 2 años de edad con uveítis crónica no infecciosa
Niños y adolescentes desde 2 años de edad con peso menor a 30 kg
La dosis habitual de Imraldi es de 20 mg en semanas alternas junto con metotrexato.
Su pediatra puede prescribir una dosis inicial de 40 mg que puede ser administrada una semana antes de empezar con la pauta habitual.
Niños y adolescentes desde 2 años de edad con un peso de 30 kg o más
La dosis habitual de Imraldi es de 40 mg en semanas alternas junto con metotrexato.
Su médico puede prescribir una dosis inicial de 80 mg que puede ser administrada una semana antes de empezar con la pauta habitual.
Forma y vía de administración
Imraldi se inyecta bajo la piel (vía subcutánea). Para instrucciones de uso, consulte la sección 7.
Si usa más Imraldi del que debe:
Si accidentalmente se inyecta Imraldi con más frecuencia de la que debiera, debe informar a su médico o farmacéutico de que ha usado más de lo necesario. Siempre lleve la caja del medicamento consigo, incluso si está vacía.
Si olvidó usar Imraldi:
Si olvida administrarse una inyección, debe inyectarse la siguiente dosis de Imraldi tan pronto como lo recuerde. Después se administrará la siguiente dosis como habitualmente, como si no se hubiese olvidado una dosis.
Si interrumpe el tratamiento con Imraldi:
La decisión de dejar de usar Imraldi debe ser discutida con su médico. Sus síntomas pueden volver tras la interrupción del tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. La mayoría de los efectos adversos son leves a moderados. Sin embargo, algunos pueden ser graves y requerir tratamiento. Los efectos adversos pueden aparecer hasta 4 meses después o más desde la última inyección de Imraldi.
Busque atención médica urgentementesi nota cualquiera de los siguientes efectos:
- erupción grave, urticaria u otros signos de reacción alérgica;
- hinchazón de la cara, manos, pies;
- dificultad para respirar, tragar;
- falta de aliento al hacer ejercicio o al estar tumbado, hinchazón de pies.
Póngase en contacto con su médico tan pronto como sea posiblesi nota alguno de los siguientes efectos:
- signos de infección tales como fiebre, ganas de vomitar, heridas, problemas dentales, sensación de quemazón al orinar;
- sensación de debilidad o cansancio;
- tos;
- hormigueo;
- entumecimiento;
- visión doble;
- debilidad en brazos o piernas;
- una protuberancia o una herida abierta que no se cura;
- signos y síntomas de alteraciones en la sangre como fiebre persistente, cardenales, hemorragias y palidez.
Los síntomas descritos anteriormente pueden ser signos de los efectos adversos listados a continuación, que se han observado con adalimumab:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- reacciones en el lugar de inyección (incluyendo dolor, hinchazón, enrojecimiento o picor);
- infecciones del tracto respiratorio inferior (incluyendo resfriado, moqueo, sinusitis, neumonía);
- dolor de cabeza;
- dolor abdominal (vientre);
- náuseas y vómitos;
- sarpullido;
- dolor en los músculos.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- infecciones graves (incluyendo envenenamiento sanguíneo y gripe);
- infecciones intestinales (incluyendo gastroenteritis);
- infecciones de la piel (incluyendo celulitis y herpes);
- infección de oído;
- infecciones de la boca (incluyendo infección dental y dolor frío);
- infecciones en el sistema reproductor;
- infección del tracto urinario;
- infecciones por hongos;
- infección en las articulaciones;
- tumores benignos;
- cáncer de piel;
- reacciones alérgicas (incluyendo alergia estacional);
- deshidratación;
- cambios de humor (incluyendo depresión);
- ansiedad;
- dificultad para dormir;
- alteraciones sensoriales como hormigueo, escozor o entumecimiento;
- migraña;
- síntomas de compresión de la raíz nerviosa (incluyendo dolor en la parte baja de la espalda y la pierna);
- alteraciones visuales;
- inflamación del ojo;
- inflamación del párpado e hinchazón del ojo;
- vértigo (sensación de que la habitación da vueltas);
- sensación de pulso acelerado;
- alta presión sanguínea;
- rubor;
- hematoma (una hinchazón palpable con sangre coagulada);
- tos;
- asma;
- dificultad para respirar;
- sangrado gastrointestinal;
- dispepsia (indigestión, hinchazón y ardor);
- reflujo ácido;
- síndrome del ojo seco (incluyendo sequedad en ojos y boca);
- picores;
- sarpullido con picor;
- moratones;
- inflamación de la piel (como eczema);
- rotura de uñas de las manos y los pies;
- aumento de la transpiración;
- pérdida de pelo;
- psoriasis de nueva aparición o empeoramiento de la psoriasis existente;
- espasmos musculares;
- sangre en orina;
- problemas renales;
- dolor de pecho;
- edema (acumulación de líquido en el organismo que provoca la hinchazón del tejido afectado);
- fiebre;
- disminución de plaquetas en sangre, lo que incrementa el riesgo de sangrado o moratones;
- problemas de cicatrización.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- infecciones oportunistas (que incluyen tuberculosis y otras infecciones que ocurren cuando la resistencia a la enfermedad disminuye);
- infecciones neurológicas (incluyendo meningitis viral);
- infecciones del ojo;
- infecciones bacterianas;
- diverticulitis (inflamación e infección del intestino grueso);
- cáncer, incluido el cáncer del sistema linfático (linfoma) y el melanoma (un tipo de cáncer de la piel);
- alteraciones inmunológicas que pueden afectar a los pulmones, piel y ganglios linfáticos (más frecuentemente como una enfermedad llamada sarcoidosis);
- vasculitis (inflamación de los vasos sanguíneos);
- temblor;
- neuropatía (lesión nerviosa);
- derrame cerebral;
- pérdida de oído, zumbidos;
- sensación de pulso irregular como brincos;
- problemas del corazón que pueden causar dificultad para respirar o hinchazón de tobillos;
- infarto de miocardio;
- saco en la pared de una arteria mayor, inflamación y coagulación en una vena, bloqueo de un vaso sanguíneo;
- enfermedades pulmonares que pueden causar dificultad para respirar (incluyendo inflamación);
- embolia pulmonar (bloqueo de una arteria del pulmón);
- derrame pleural (almacenamiento anormal de fluido en el espacio pleural);
- inflamación del páncreas que causa un dolor grave en el abdomen y la espalda;
- dificultad para tragar;
- edema facial;
- inflamación de la vesícula, piedras en la vesícula;
- grasa en el hígado (acumulación de grasa en las células hepáticas);
- sudores nocturnos;
- cicatrices;
- crisis muscular anormal;
- lupus eritematoso sistémico (incluyendo inflamación de la piel, corazón, pulmones, articulaciones y otros órganos);
- interrupciones del sueño;
- impotencia;
- inflamaciones.
Raros(pueden afectar hasta 1 de cada 1000 personas):
- leucemia (cáncer que afecta a la sangre y la médula ósea);
- reacción alérgica grave con shock;
- esclerosis múltiple;
- alteraciones nerviosas (como inflamación del nervio óptico hacia el ojo, y síndrome de Guillain- Barré, una enfermedad que puede provocar debilidad muscular, sensaciones anormales, hormigueo en los brazos y la parte superior del cuerpo);
- parada cardiaca;
- fibrosis pulmonar (cicatriz en el pulmón);
- perforación intestinal;
- hepatitis;
- reactivación del virus de la hepatitis B;
- hepatitis autoinmune (inflamación del hígado causada por el propio sistema inmunológico del cuerpo);
- vasculitis cutánea (inflamación de los vasos sanguíneos en la piel);
- síndrome de Stevens-Johnson (los síntomas tempranos incluyen malestar, fiebre, dolor de cabeza y sarpullido);
- edema facial asociado con reacciones alérgicas;
- eritema multiforme (erupción inflamatoria en la piel);
- síndrome similar al lupus;
- angioedema (inflamación localizada de la piel);
- reacción liquenoide en la piel (sarpullido rojizo-morado con picor).
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- linfoma hepatoesplénico de células T (cáncer sanguíneo raro que a menudo es mortal);
- carcinoma de células de Merkel (un tipo de cáncer de piel);
- sarcoma de Kaposi, un cáncer poco común relacionado con la infección por el virus del herpes humano 8. El sarcoma de Kaposi suele manifestarse con mayor frecuencia como lesiones cutáneas de color purpura;
- fallo hepático;
- empeoramiento de una enfermedad llamada dermatomiositis (visto como erupción cutánea acompañada de debilidad muscular);
- aumento de peso (para la mayoría de pacientes, el aumento de peso fue reducido).
Algunos efectos adversos con adalimumab observados en los ensayos clínicos no tienen síntomas y sólo pueden ser identificados mediante un análisis de sangre. Estos incluyen:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- bajo recuento sanguíneo de células blancas;
- bajo recuento sanguíneo de células rojas;
- aumento de lípidos en sangre;
- aumento de enzimas hepáticas.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- alto recuento sanguíneo de células blancas;
- bajo recuento sanguíneo de plaquetas;
- aumento de ácido úrico en sangre;
- valores anormales de sodio en sangre;
- bajo nivel de calcio en sangre;
- bajo nivel de fosfato en sangre;
- azúcar en sangre alto;
- valores altos de lactato deshidrogenasa en sangre;
- presencia de autoanticuerpos en sangre;
- bajo nivel de potasio en sangre.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- valores de bilirrubina altos (análisis de función hepática).
Raros(pueden afectar hasta 1 de cada 1000 personas):
- recuentos bajos en sangre para células blancas, células rojas y plaquetas.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte con su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Imraldi
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la caja después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar.
Conservar la pluma precargada en el embalaje exterior para protegerla de la luz.
Almacenamiento alternativo:
Cuando sea necesario (por ejemplo cuando esté de viaje), puede almacenar una pluma precargada individual de Imraldi temperatura ambiente (hasta 25 ºC) durante un periodo máximo de 28 días (asegúrese de protegerla de la luz). Una vez que se ha sacado de la nevera para almacenar la pluma a temperatura ambiente, debe usarla en los siguientes 28 días o desecharla, incluso si la vuelve a meter en la nevera.
Debe anotar la fecha en la que retiró la pluma de la nevera, y la fecha después de la cual debe desechar la pluma.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Imraldi
- El principio activo es adalimumab.
- Los demás componentes son: citrato de sodio, ácido cítrico monohidrato, histidina, clorhidrato monohidrato de histidina, sorbitol, polisorbato 20 y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Imraldi 40 mg solución inyectable en pluma precargada se suministra como 0,4 ml de solución de transparente a opalescente y de incolora a marrón pálido.
Imraldi está disponible en envases que contienen 1, 2, 4 o 6 pluma(s) precargada(s) con una jeringa precargada dentro (vidrio tipo I) con una aguja de acero inoxidable, un protector de aguja rígido, un émbolo de goma para el uso por el paciente y 2, 2, 4 o 6 toallitas con alcohol incluidas en el envase correspondiente.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Samsung Bioepis NL B.V.
Olof Palmestraat 10
2616 LR Delft
Países Bajos
Responsable de la fabricación
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Países Bajos
Samsung Bioepis NL B.V.
Olof Palmestraat 10
2616 LR Delft
Países Bajos
Pueden solicitar más información respecto a este medicamento, dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Biogen Belgium NV/S.A Tél/Tel: + 32 2 2191218 | Lietuva Biogen Lithuania UAB Tel: +370 5 259 6176 |
Ewopharma AG Representative Office Te?.: + 35929621200 | Luxembourg/Luxemburg Biogen Belgium NV/SA Tél/Tel: +35 2 2 2191218 |
Ceská republika Biogen (Czech Republic) s.r.o. Tel: + 420 255 706 200 | Magyarország Biogen Hungary Kft. Tel.: + 36 1 899 9880 |
Danmark Biogen (Denmark) A/S Tlf: + 45 77 41 57 57 | Malta Pharma.MT Ltd Tel: + 356 21337008 |
Deutschland Biogen GmbH Tel: + 49 (0) 89 99 6170 | Nederland Biogen Netherlands B.V. Tel: + 31 20 542 2000 |
Eesti Biogen Estonia OÜ Tel: + 372 618 9551 | Norge Biogen Norway AS Tlf: + 47 23 40 01 00 |
Ελλáδα Genesis Pharma S.A. Τηλ: + 30 2108771500 | Österreich Biogen Austria GmbH Tel: + 43 1 484 46 13 |
España Biogen Spain, S.L. Tel: + 34 91 310 7110 | Polska Biogen Poland Sp. z o.o. Tel.: + 48 22 351 51 00 |
France Biogen France SAS Tél: + 33 (0)1 776 968 14 | Portugal Biogen Portugal Sociedade Farmacêutica, Unipessoal, Lda Tel: + 351 21 318 8450 |
Hrvatska Ewopharma d.o.o Tel: + 385 (0)1 6646 563 | România Ewopharma AG Representative Office Tel: + 40 212601344 |
Ireland Biogen Idec (Ireland) Ltd. Tel: +353 (0)1 463 7799 | Slovenija Biogen Pharma d.o.o. Tel: + 386 1 511 02 90 |
Ísland Icepharma hf. Sími: + 354 6540 8000 | Slovenská republika Biogen Slovakia s.r.o. Tel: + 421 2 323 340 08 |
Italia Biogen Italia s.r.l. Tel: + 39 2 584 99 010 | Suomi/Finland Biogen Finland Oy Puh/Tel: + 358 207 401 200 |
Κúπρος Genesis Pharma (Cyprus) Ltd Τηλ: + 357 22 76 57 15 | Sverige Biogen Sweden AB Tel: +46 8 594 113 60 |
Latvija Biogen Latvia SIA Tel: + 371 68 688 158 |
Fecha de la última revisión de este prospecto: 03/2025
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- Instrucciones de uso
Siga atentamente estas instrucciones de uso y en poco tiempo desarrollará una rutina para poner la inyección con seguridad.
- Antes de ponerse la inyección usted mismo pida a su médico o enfermero que le muestre cómo usar la pluma precargada. Su médico o enfermero debe asegurarse de que usted puede usar correctamente la pluma.
Pluma precargada con una sola dosis
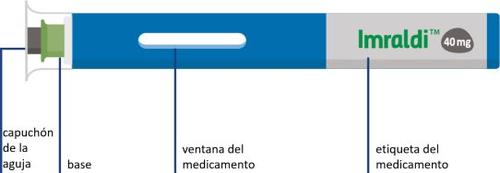
Su pluma precargada no tiene botón.
La aguja está oculta debajo de la base verde. La inyección se iniciar de manera automática cuando aprieta firmemente la pluma precargada sobre la piel.
Cuidado de su pluma precargada
Conservación de la pluma
- Conserve la pluma en el frigorífico, pero no la congele.
- Mantenga la pluma en su caja y protegida de la luz.
- Mantenga la pluma fuera de la vista y del alcance de los niños.
Eliminación de la pluma
- Use cada pluma una sola vez. Nunca reutilice una pluma.
- Tire la pluma usada en un contenedor especial tal y como le explicó su médico, enfermero o farmacéutico.
Precauciones
- Si se le ha caído la pluma CON el capuchón puede usar la pluma.
Si se le ha caído la pluma SIN el capuchón, no la use. Es posible que la auja esté sucia o dañada.
- No use una pluma dañada.
Cuidado del lugar de la inyección
- Elija una zona con grasa para poner la inyección
Las zonas con grasa, como el abdomen, son por lo general los mejores lugares para poner la inyección. Las zonas con grasa son buenas para insertar correctamente la aguja.
- Utilice un lugar diferente para cada nueva inyección
Cuando elija un lugar para la inyección escoja un sitio que no haya usado recientemente para que no le duela ni aparezcan hematomas.
Cómo inyectar con su pluma precargada
- Reúna los materiales necesarios para la inyección

Coloque la pluma precargada y las toallitas con alcohol sobre una superficie limpia y seca.
- ¡No olvide lavarse las manos!
- ¡No quite todavía el capuchón!
- Espere entre 15-30 minutos
Espere entre 15-30 minutos para que la pluma precargada alcance la temperatura ambiente; esto ayuda a reducir el dolor durante la inyección.
- ¡No quite todavía el capuchón!
- Compruebe el medicamento y la fecha de caducidad
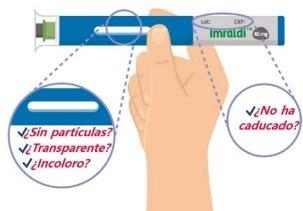
Asegúrese siempre de que el medicamento es de transparente a opalescente, de incoloro a marrón pálido, no presenta partículas y no ha caducado. Si el medicamento no es de transparente a opalescente, de incoloro a marrón pálido, tiene partículas o ha caducado, no lo use.
Es posible que vea una o más burbujas de aire, es normal y no es motivo para desechar el medicamento.
- ¡No quite todavía el capuchón!
- Elija un lugar de inyección y limpie la piel
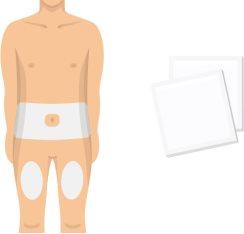
Elija un lugar de inyección en su cuerpo. Las zonas más adecuadas son el abdomen (excepto la zona alrededor del ombligo) y los muslos.
Limpie el lugar de la inyección con una toallita con alcohol. No vuelva a tocar la zona antes de la inyección.
- Evite las zonas de la piel doloridas, magullada, con cicatrices, escamas o placas rojas.
- Quite el capuchón transparente de la aguja
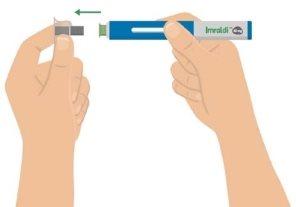
Quite con cuidado de la pluma el capuchón transparente de la aguja con núcleo metálico.
Es normal que salgan unas gotas de líquido de la aguja.
Si quita el capuchón de la aguja antes de estar preparado para la inyección, no lo vuelva a ponerporque podría doblar o dañar la aguja. Podría pincharse accidentalmente o desperdiciar el medicamento.
- Coloque la base verde, apriete y mantenga la presión
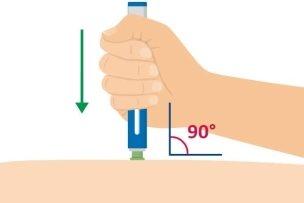
Coloque la base verde en vertical (90 grados) sobre la piel, y presione la pluma precargada hacia abajo con firmeza para que se inicie la inyección.
La inyección comienza cuando usted presiona hacia abajo. Puede que oiga un 1er clic.
- Mantenga la presión
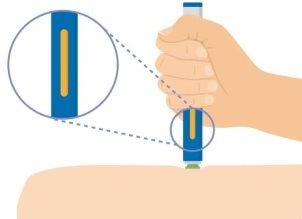
Mantenga la pluma contra la piel hasta que el indicador amarillo llene la ventana del medicamento y deje de moverse.
Varios segundos después puede que oiga un 2º clic.
- Confirme la finalización y deseche

Después de inyectar el medicamento Imraldi confirme que toda la ventana está amarilla.
Deseche la pluma usada en un contenedor especial como le indicó su médico, enfermero o farmacéutico.
¿No está seguro de haber administrado su dosis completa? Póngase en contacto con su médico, enfermero o farmacéutico.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a IMRALDI 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: AdalimumabFabricante: Amgen Europe B.V.Requiere recetaForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: AdalimumabFabricante: Amgen Europe B.V.Requiere recetaForma farmacéutica: INYECTABLE, 40 mgPrincipio activo: AdalimumabFabricante: Amgen Europe B.V.Requiere receta
Médicos online para IMRALDI 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de IMRALDI 40 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






