HYQVIA 100 mg/mL SOLUTION FOR INFUSION


How to use HYQVIA 100 mg/mL SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
HyQvia100mg/ml, solution for infusion
human normal immunoglobulin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is HyQvia and what is it used for
- What you need to know before you use HyQvia
- How to use HyQvia
- Possible side effects
- Storage of HyQvia
- Contents of the pack and further information
1. What is HyQvia and what is it used for
What is HyQvia
HyQvia contains 2 solutions for infusion (drip) under the skin (subcutaneous infusion or SC). It is supplied in a pack that contains:
- a vial of human normal immunoglobulin 10% (the active substance)
- a vial of recombinant human hyaluronidase (a substance that helps human normal immunoglobulin 10% to reach the blood).
Human normal immunoglobulin 10% belongs to a class of medicines called “human normal immunoglobulins”. Immunoglobulins are also antibodies and are found in the blood of healthy people. Antibodies are part of the immune system (the body's natural defenses) and help the body to fight infections.
How HyQvia works
Recombinant human hyaluronidase is a protein that facilitates the infusion (by drip) of immunoglobulins under the skin and their entry into the circulatory system.
The vial of immunoglobulins is prepared from the blood of healthy people. Immunoglobulins are produced by the human immune system. They help your body to fight infections caused by bacteria and viruses or to maintain the balance of the immune system (which is known as immunomodulation). The medicine works in the same way as the immunoglobulins that are naturally present in the blood.
What HyQvia is used for
Replacement therapy in adults and children (from 0 to 18 years of age)
HyQvia is used in patients with a weak immune system, who do not have enough antibodies in the blood and are prone to infections, including the following groups:
- patients who are born with an inability or reduced ability to produce antibodies (primary immunodeficiencies),
- patients who suffer from severe or recurrent infections due to a weakened immune system resulting from other conditions or treatments (secondary immunodeficiencies).
Regular and sufficient doses of HyQvia can increase the abnormally low levels of immunoglobulins in your blood to normal levels (replacement therapy).
Immunomodulatory therapy in adults, children, and adolescents (from 0 to 18 years of age)
- HyQvia is used in adult, child, and adolescent patients (from 0 to 18 years) with chronic inflammatory demyelinating polyneuropathy (CIDP), a type of autoimmune disease. CIDP is characterized by chronic inflammation of the peripheral nerves that causes muscle weakness and/or numbness, mainly in the legs and arms. It is believed that the body's own defense system attacks the peripheral nerves and causes nerve damage and inflammation. It is believed that the immunoglobulins present in HyQvia help to protect the nerves from damage caused by the immune system.
2. What you need to know before you use HyQvia
Do not inject or infuse HyQvia
- if you are allergic to immunoglobulins, hyaluronidase, recombinant hyaluronidase, or any of the other components of this medicine (listed in section 6, “Contents of the pack and further information”)
- if you have antibodies against immunoglobulin A (IgA) in the blood. This may occur if you have an IgA deficiency. As HyQvia contains traces of IgA, you may experience an allergic reaction
- into a blood vessel (intravenously) or into a muscle (intramuscularly).
Warnings and precautions
Talk to your doctor or nurse before you start using HyQvia.
- Before treatment, tell your doctor or healthcare professional if any of the following apply to you:
- You or your child may be allergic to immunoglobulins and not know it. Allergic reactions, such as a sudden drop in blood pressure or anaphylactic shock (a sudden drop in blood pressure along with other symptoms such as swelling of the throat, difficulty breathing, and skin rash), are rare but can occur even if you have not had problems before with similar treatments. You are at greater risk of experiencing allergic reactions if you have an IgA deficiency with anti-IgA antibodies. The signs or symptoms of these rare allergic reactions include:
- dizziness, lightheadedness, or loss of consciousness,
- skin rash and itching, swelling of the mouth or throat, difficulty breathing, wheezing (a whistling sound when breathing),
- abnormal heart rate, chest pain, bluish discoloration of lips or fingers and toes,
- blurred vision
- If you notice any of these signs during infusion, tell your doctor or nurse immediately. They will decide whether to reduce the infusion rate or stop it completely.
Your doctor or nurse will infuse recombinant human hyaluronidase (HY) followed by immunoglobulin (Ig) slowly and carefully, and will monitor you during the 1st infusion to detect and treat any allergic reaction immediately.
- Your doctor will be particularly careful if you are overweight, are an elderly patient, have diabetes, have been bedridden for a long period, have high blood pressure, have low blood volume (hypovolemia), have vascular problems (vascular diseases), have an increased tendency for blood to clot (thrombophilia or thrombotic episodes), or have a disease that causes the blood to be thicker (hyperviscous blood). In these circumstances, immunoglobulins may increase the risk of heart attack (myocardial infarction), stroke, blood clots in the lungs (pulmonary embolism), or blockage of a blood vessel in the leg, although this is very rare.
- If you notice any of these signs and symptoms during infusion, including shortness of breath, pain, swelling of a limb, and chest pain, tell your doctor or nurse immediately. They will decide whether to reduce the infusion rate or stop it completely.
Your doctor or nurse will monitor you closely during infusions to detect and treat any thromboembolic events immediately.
- You will receive this medicine in high doses over 1 or 2 days, and if you belong to blood groups A, B, or AB and have an underlying inflammatory disease. In these circumstances, it has been reported that immunoglobulins increase the risk of destruction of red blood cells (hemolysis).
- There have been reports of inflammation of the membranes surrounding the brain and spinal cord (aseptic meningitis syndrome) related to treatment with immunoglobulins.
- If you notice any of these signs and symptoms after infusion, including severe headache, stiff neck, dizziness, fever, photophobia, nausea, and vomiting, tell your doctor or nurse immediately.
Your doctor will decide whether further tests are needed and whether treatment with HyQvia should continue.
Infusion rate
It is very important to infuse the medicine at the correct rate. Your doctor or nurse will advise you on the correct infusion rate for when you infuse HyQvia at home (see section 3, “How to use HyQvia”).
Monitoring during infusion
Certain side effects may occur more frequently if:
- you are receiving HyQvia for the first time
- you have received another immunoglobulin and have switched to HyQvia
- it has been a long time (e.g., more than 2 or 3 infusion intervals) since you last received HyQvia.
- In such cases, you will be closely monitored during the 1st infusion and during the 1st hour after the infusion has finished.
In other cases, you will be closely monitored during infusion and for at least 20 minutes after you have received the 1st infusions of HyQvia.
Home treatment
Before starting home treatment, you will be assigned a caregiver. You and your caregiver will be trained to detect the first signs of side effects, especially allergic reactions. This caregiver will help you to monitor any side effects. During infusion, you should monitor whether the first signs of side effects occur (for more details, see section 4, “Possible side effects”).
- If you notice any side effect, you or your caregiver should stop the infusion immediately and contact a doctor.
- If you experience a serious side effect, you or your caregiver should seek emergency treatment immediately.
Spread of localized infections
Do not infuse HyQvia into or around an infected or swollen and red area of the skin, as this could spread the infection.
No long-term (chronic) changes in the skin were observed in clinical studies. You should tell your doctor about any long-term inflammation, lumps (nodules), or swelling that appears at the infusion site and lasts for more than a few days.
Effects on blood tests
HyQvia contains many different antibodies, some of which may interfere with blood tests (serological tests).
- Before you have a blood test, tell your doctor about your treatment with HyQvia.
Information about the source material of HyQvia
The human normal immunoglobulin 10% of HyQvia and the human serum albumin (a component of the recombinant human hyaluronidase) are produced from human plasma (the liquid part of the blood). When medicines are made from human blood or plasma, a number of measures are taken to prevent the possible transmission of infections to patients. These include:
- careful selection of blood and plasma donors to ensure the exclusion of donors who may be at risk of carrying infectious diseases
- testing of each donation and plasma pool for the presence of possible viruses or infections.
The manufacturers of these products also include steps in the manufacturing process to eliminate/inactivate viruses. Despite this, when medicines derived from human blood or plasma are used, the possibility of transmitting infectious agents cannot be completely ruled out. This also applies to emerging or unknown viruses and infectious agents.
The measures taken for the manufacture of HyQvia are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), and for non-enveloped viruses such as hepatitis A virus and parvovirus B19.
Immunoglobulins have not been associated with hepatitis A or parvovirus B19 infections, probably because the antibodies associated with these infections (and which are present in HyQvia) provide protection.
- It is strongly recommended that each time you are given a dose of HyQvia, the following information is recorded in the patient's diary:
- the name of the product,
- the date of administration,
- the batch number of the medicine, and
- the volume injected, the rate of administration, the number, and the location of the infusion sites.
Children and adolescents
Replacement therapy
The same indications, doses, and infusion frequency for adults apply to children and adolescents (0 to 18 years).
Immunomodulatory therapy in patients with CIDP
The safety and efficacy of HyQvia in children and adolescents (0 to 18 years of age) with CIDP have not been established.
Other medicines and HyQvia
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
Vaccines
HyQvia may reduce the effect of some vaccines, such as measles, mumps, rubella, and varicella (live virus vaccines). Therefore, after receiving HyQvia, you may need to wait up to 3 months before receiving certain vaccines. You may need to wait up to 1 year after receiving HyQvia before you can receive the measles vaccine.
- Before vaccination, tell your doctor or nurse about your treatment with HyQvia.
Pregnancy, breastfeeding, and fertility
Data on the effects of long-term use of recombinant human hyaluronidase on pregnancy, breastfeeding, and fertility are limited. HyQvia should only be used in pregnant or breastfeeding women after consulting a doctor.
Driving and using machines
During treatment with HyQvia, patients may experience side effects (e.g., dizziness or nausea) that may affect their ability to drive and use machines. If this occurs, you should wait until the reactions have disappeared.
HyQvia contains sodium
This medicine contains 5.0 to 60.5 mg of sodium (the main component of cooking/table salt) in each vial of recombinant human hyaluronidase HyQvia. This is equivalent to 0.25 to 3% of the maximum recommended daily intake of sodium for an adult.
The Ig 10% component is essentially sodium-free.
3. How to use HyQvia
Follow your doctor's administration instructions for this medication exactly. If in doubt, consult your doctor again.
HyQvia must be infused under the skin (subcutaneous or SC administration).
Your doctor or nurse will start the treatment with HyQvia, but once you have received the first infusions under medical supervision and you (and/or your caregiver) are properly trained, you can use the medication at home. You and your doctor will decide if you can use HyQvia at home. Do not start treatment with HyQvia at home until you have received complete instructions.
Dosage
Replacement therapy
Your doctor will calculate the correct dose based on your body weight, previous treatments you have received, and your response to treatment. The recommended starting dose is one that provides 400 to 800 mg of active ingredient per kg of body weight per month. Initially, you will receive a quarter of this dose at weekly intervals. Subsequent infusions will gradually increase to higher doses at 3 to 4 week intervals. Sometimes, the doctor may recommend dividing the larger doses and administering them at 2 sites at the same time. The doctor may also adjust the dose depending on your response to treatment.
Immunomodulatory therapy
Your doctor will calculate the correct dose for you based on previous treatments you have received and your response to treatment. Normally, treatment starts 1 or 2 weeks after the last subcutaneous infusion of immunoglobulin with the equivalent weekly dose calculated. Your healthcare professional may adjust the dose and frequency based on your response to treatment.
In case the daily dose is exceeded (> 120 g) or if you cannot tolerate the infusion volume of immunoglobulins, the dose can be divided and administered over several days, leaving 48 to 72 hours between doses to allow for proper absorption; the administration of hyaluronidase should also be divided accordingly.
Starting treatment
Treatment will be initiated by a doctor or nurse with experience in treating patients with a weak immune system (immunodeficiency) and PDIC to train patients for home treatment. You will be closely monitored during the infusion and for at least 1 hour after to see if you tolerate the medication well. Initially, your doctor or nurse will use a slow infusion rate and gradually increase it during the first infusion and subsequent ones. Once the doctor or nurse has found the right dose and infusion rate for you, you can administer the treatment at home.
Home treatment
Do not use HyQvia at home until you receive instructions and training from a healthcare professional.
You will be trained in:
- Aseptic infusion techniques (without germs),
- The use of an infusion pump or a continuous infusion pump (if necessary),
- Maintaining a patient diary and
- Measures to be taken in case of serious adverse reactions.
You must carefully follow your doctor's instructions regarding the dose, infusion rate, and planning when infusing HyQvia, so that the treatment works.
The following infusion rates are recommended for Ig 10% per infusion site:
Subjects<40kg | Subjects≥40kg | |||
Interval/minutes | First 2 infusions (ml/hour/infusion site) | 2 to 3 subsequent infusions (ml/hour/infusion site) | First 2 infusions (ml/hour/infusion site) | 2 to 3 subsequent infusions (ml/hour/infusion site) |
10 minutes | 5 | 10 | 10 | 10 |
10 minutes | 10 | 20 | 30 | 30 |
10 minutes | 20 | 40 | 60 | 120 |
10 minutes | 40 | 80 | 120 | 240 |
Rest of infusion | 80 | 160 | 240 | 300 |
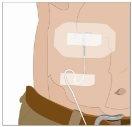
The indicated infusion rates are for a single infusion site. If the patient needs 2 or 3 infusion sites, the infusion rates can be adjusted accordingly (i.e., doubled or tripled based on the maximum infusion rate of the pump).
If a loss occurs at the infusion site
Ask your doctor, pharmacist, or nurse if a different needle size would be more suitable for you. Any change in needle size should be supervised by a doctor.
If you use more HyQvia than you should
If you think you have used more HyQvia than you should, consult your doctor as soon as possible.
If you forget to use HyQvia
Do not administer a double dose of HyQvia to make up for forgotten doses. If you think you have forgotten a dose, consult your doctor as soon as possible.
If you have any other questions about using this medication, ask your doctor, pharmacist, or nurse.
The following section provides detailed instructions on use.
| |
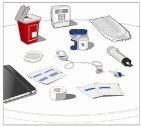
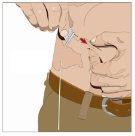
Prepare all materialsfor the infusion. These include: HyQvia double vial unit(s), infusion materials (subcutaneous needle, solution container (bag or syringe), sterile dressing and tape, pump tubes, transfer devices, syringes, gauze, and tape), sharps container, pump, and treatment logbook, as well as any other materials that may be necessary. |
|
| |
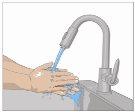
Wash your hands thoroughly. Place all necessary materials and open them according to your healthcare professional's instructions. |
|
|
|
|
|
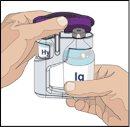
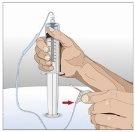
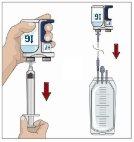
IF using the push method to administer (HY):
IF using the pump method to administer (HY):
|
|
|
|
Follow the manufacturer's instructions when preparing the pump. | |
|
|
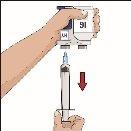
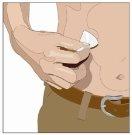
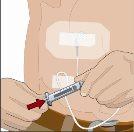
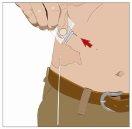
| 90-degree angle in relation to the skin
|
|
|
|
|
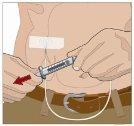
Divide the contents equally among all sites if using more than one site. IF using the push method to administer HY:
IF using the pump method to administer HY:
|
|
After infusing all the contents of the smaller syringe (recombinant human hyaluronidase), remove the syringe from the needle connector or the pump tubes. Connect the pump tubes to the container or the vial of Ig or the larger syringe containing the normal human immunoglobulin 10% to the needle. Administer the normal human immunoglobulin 10% with a pump at the rates indicated by your healthcare professional and start the infusion. | |
| |
|
|
|
4. Possible Adverse Effects
Like all medicines, this medicine may cause adverse effects, although not all people suffer from them. Some adverse effects, such as headache, chills, or body aches, can be reduced by decreasing the infusion rate.
Severe Adverse Effects
The infusion of medicines like HyQvia may occasionally cause severe allergic reactions, although rare. You may experience a sudden drop in blood pressure and, in isolated cases, anaphylactic shock. Doctors are aware of these possible adverse effects and will monitor you during and after initial infusions.
The typical signs or symptoms include:
dizziness, drowsiness, or loss of consciousness, skin rash and itching, swelling of the mouth or throat, difficulty breathing, wheezing (a whistling sound when breathing), abnormal heart rate, chest pain, bluish discoloration of lips or fingers and toes, blurred vision.
- If you notice any of these signs during the infusion, inform your doctor or nurse immediately.
- When using HyQvia at home, you must perform the infusion in the presence of an assigned caregiver who will help you monitor allergic reactions, stop the infusion, and seek help if necessary.
- Also, see section 2 of this prospectus on the risk of allergic reactions and the use of HyQvia at home.
Very Common Adverse Effects (may occur in more than 1 in 10 infusions):
Local reactions at the infusion site (including all infusion sites listed below). These reactions usually disappear within a few days.
Common Adverse Effects (may occur in up to 1 in 10 infusions):
- headache
- discomfort (nausea)
- abdominal pain/pain on palpation of the abdomen
- skin redness (erythema)
- reactions at the infusion site, including pain, discomfort, pain on palpation, redness, swelling, and itching
- feeling of heat, fever
- weakness (asthenia), fatigue, lack of energy (lethargy), and general feeling of discomfort
Uncommon Adverse Effects (may occur in up to 1 in 100 infusions):
- dizziness
- migraine
- numbness, tingling, or prickling sensations (paresthesia)
- tremor
- rapid heartbeat (tachycardia)
- high blood pressure (hypertension)
- abdominal swelling (abdominal distension)
- diarrhea
- vomiting
- rash
- itching (pruritus)
- itchy rash (urticaria)
- muscle pain (myalgia)
- joint pain (arthralgia)
- back pain
- pain in the limbs (including discomfort in the limbs)
- musculoskeletal chest pain
- joint stiffness
- reactions at the infusion site (such as color change, bruising, redness [hematoma], bleeding [hemorrhage], puncture of the blood vessel, lump [nodule], induration, swelling [edema], chills, burning sensation, rash)
- genital swelling
- Rare Adverse Effects (may occur in up to 1 in 1,000 infusions):
- stroke
- low blood pressure (hypotension)
- difficulty breathing (dyspnea)
- groin pain
- brown urine (hemosiderinuria)
- excessive sweating (hyperhidrosis)
- inflammation of the infusion site
- heat at the infusion site
- numbness, tingling, and prickling sensations at the infusion site (paresthesia at the infusion site)
- positive Coombs test result
Frequency Not Known (cannot be estimated from the available data):
- inflammation of the membranes surrounding the brain and spinal cord (aseptic meningitis)
- allergic reactions (hypersensitivity)
- loss at the infusion site
- pseudo-flu syndrome (flu-like illness)
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of HyQvia
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the label and carton after CAD. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C - 8°C). Do not freeze.
Do not shake.
Keep the vials in the outer packaging to protect them from light.
Do not use this medicine if you notice that the solutions appear turbid or have particles or sediments.
After opening, discard any unused solution from the vials.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of HyQvia
HyQvia is a dual vial unit that contains:
- a solution of recombinant human hyaluronidase (Step 1 of HyQvia/Infuse first) and
- a solution of normal human immunoglobulin 10% (Step 2 of HyQvia/Infuse second).
The content of each vial is described below:
- Recombinant human hyaluronidase
This vial contains recombinant human hyaluronidase.
The other components are sodium chloride, sodium phosphate, human albumin, disodium ethylenediaminetetraacetic acid (EDTA), calcium chloride, and water for injectable preparations (see also section 2, "HyQvia contains sodium").
- Normal human immunoglobulin 10%
One milliliter of the solution in this vial contains 100 mg of normal human immunoglobulin, of which at least 98% is immunoglobulin G (IgG).
The active ingredient of HyQvia is normal human immunoglobulin. This medicine contains traces of immunoglobulin A (IgA) (no more than 140 micrograms/ml, 37 micrograms on average).
The other components of this vial are glycine and water for injectable preparations.
Appearance of the Product and Package Contents
HyQvia 100 mg/ml solution for subcutaneous infusion (infusion under the skin).
HyQvia is supplied in a package that contains:
- a glass vial of recombinant human hyaluronidase and
- a glass vial of normal human immunoglobulin 10%.
The recombinant human hyaluronidase is a clear and colorless solution.
The normal human immunoglobulin 10% is a clear and colorless or slightly yellowish solution.
The following package sizes are available:
Recombinant Human Hyaluronidase | Normal Human Immunoglobulin 10% | |
Volume (ml) | Protein (g) | Volume (ml) |
1.25 | 2.5 | 25 |
2.5 | 5 | 50 |
5 | 10 | 100 |
10 | 20 | 200 |
15 | 30 | 300 |
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Baxalta Innovations GmbH
Industriestrasse 67
A-1221 Vienna
Austria
Manufacturer:
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
B-7860 Lessines
Belgium
You can request more information about this medicine by contacting the local representative of the Marketing Authorization Holder:
Takeda Farmacéutica España, S.A.
Tel: +34 917 90 42 22
Date of the Last Revision of this Prospectus: 05/2024.
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HYQVIA 100 mg/mL SOLUTION FOR INFUSIONDosage form: INJECTABLE, 165 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Octapharma S.A.Prescription requiredDosage form: INJECTABLE, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Baxalta Innovations GmbhPrescription requiredDosage form: INJECTABLE, 200 mg/mlActive substance: immunoglobulins, normal human, for extravascular adm.Manufacturer: Csl Behring GmbhPrescription required
Online doctors for HYQVIA 100 mg/mL SOLUTION FOR INFUSION
Discuss questions about HYQVIA 100 mg/mL SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions