
FIXAPROST 50 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION EN ENVASES UNIDOSIS

Cómo usar FIXAPROST 50 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION EN ENVASES UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el paciente
Fixaprost 50 microgramos/ml + 5 mg/ml colirio en solución en envase unidosis
latanoprost / timolol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Fixaprost y para qué se utiliza
- Qué necesita saber antes de empezar a usar Fixaprost
- Cómo usar Fixaprost
- Posibles efectos adversos
5 Conservación de Fixaprost
- Contenido del envase e información adicional
1. Qué es Fixaprost y para qué se utiliza
Fixaprost contiene dos principios activos: latanoprost y timolol. Latanoprost pertenece a un grupo de medicamentos conocidos como análogos de las prostaglandinas. Timolol pertenece a un grupo de medicamentos llamados betabloqueantes. Latanoprost actúa aumentando la salida natural de líquido desde el interior del ojo al torrente sanguíneo. Timolol actúa reduciendo la formación de líquido en el ojo.
Fixaprost se utiliza para reducir la presión del ojo en caso de que padezca unas enfermedades conocidas como glaucoma de ángulo abierto o hipertensión ocular. Ambas enfermedades están relacionadas con un aumento de la presión dentro del ojo, lo que puede llegar a afectar a la visión. Su médico normalmente le recetará Fixaprost cuando otros medicamentos no hayan funcionado adecuadamente.
2. Qué necesita saber antes de empezar a usar Fixaprost
Fixaprost puede utilizarse en adultos (incluyendo pacientes de edad avanzada) pero no se recomienda su uso en menores de 18 años de edad.
No use Fixaprost:
- Si es alérgico (hipersensible) a latanoprost, timolol, betabloqueantes o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene o ha tenido en el pasado problemas respiratorios como asma, bronquitis obstructiva crónica grave (enfermedad grave del pulmón que puede causar pitos dificultad para respirar y/o tos de larga duración).
- Si tiene problemas graves de corazón o trastornos de la frecuencia cardiaca.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Fixaprost, si tiene o ha tenido en el pasado:
- Enfermedad coronaria cardiaca (los síntomas pueden incluir dolor de pecho u opresión, respiración entrecortada o asfixia), insuficiencia cardiaca, tensión arterial baja.
- Alteraciones en la frecuencia cardiaca como por ejemplo latido lento del corazón.
- Problemas en la respiración, asma o enfermedad pulmonar obstructiva crónica.
- Enfermedades caracterizadas por una escasa circulación de la sangre (como la enfermedad de Raynaud o el síndrome de Raynaud).
- Diabetes, ya que el timolol puede enmascarar los signos y síntomas de niveles bajos de azúcar en sangre.
- Hiperactividad de la glándula tiroides ya que el timolol puede enmascarar signos y síntomas.
- Si va a sufrir una intervención quirúrgica ocular (incluyendo una operación de cataratas) o ha tenido algún tipo de cirugía ocular.
- Si padece problemas en los ojos (tales como dolor, irritación o inflamación en el ojo o visión borrosa).
- Si padece de ojo seco.
- Si utiliza lentes de contacto. Puede seguir utilizando Fixaprost, pero ha de seguir las instrucciones que se incluyen en la sección 3 para usuarios de lentes de contacto.
- Si padece de angina (en particular de un tipo conocido como angina de Prinzmetal).
- Si padece reacciones alérgicas graves que habitualmente requieren tratamiento hospitalario.
- Si ha sufrido o está sufriendo una infección vírica en el ojo causada por el virus del herpes simple (VHS).
Informe a su médico de que está utilizando Fixaprost antes de someterse a una operación, ya que timolol puede modificar los efectos de algunos medicamentos utilizados durante la anestesia.
Uso de Fixaprostconotros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento, incluyendo colirios y medicamentos obtenidos sin receta.
Fixaprost puede afectar o ser afectado por otros medicamentos que esté utilizando, incluyendo otros colirios para el tratamiento del glaucoma. Informe a su médico si está utilizando o tiene intención de utilizar medicamentos para disminuir la tensión arterial, medicamentos para el corazón o medicamentos para tratar la diabetes.
En particular, hable con su médico o farmacéutico si está tomando alguno de los siguientes tipos de medicamentos:
- Prostaglandinas, análogos de prostaglandinas o derivados de prostaglandinas.
- Betabloqueantes.
- Epinefrina.
- Medicamentos utilizados para tratar la tensión arterial elevada tales como bloqueantes de los canales de calcio orales, guanetidina, antiarrítmicos, glucósidos digitálicos o parasimpaticomiméticos.
- Quinidina (utilizada para tratar enfermedades del corazón y determinados tipos de malaria).
- Antidepresivos como la fluoxetina y la paroxetina.
Uso de Fixaprost con alimentos y bebidas
Las comidas, alimentos y bebidas habituales no tienen efecto sobre cuándo o cómo debe utilizar Fixaprost.
Embarazo,lactanciay fertilidad
No utilice Fixaprostsi está embarazada o en periodo de lactancia.
Fixaprost podría pasar a la leche.
En los estudios en animales no se ha encontrado que latanoprost ni timolol ejerzan ningún efecto sobre la fertilidad masculina o femenina.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Al utilizar Fixaprost puede aparecer visión borrosa durante un periodo de tiempo breve. Si esto le sucede, no conduzca ni utilice herramientas o máquinas hasta que su visión vuelva a ser nítida de nuevo.
Fixaprost contiene hidroxiestearato de macrogolglicerol(derivado del aceite de ricino) que puede causar reacciones cutáneas.
Uso en deportistas
Este medicamento contiene timolol que puede producir un resultado positivo en las pruebas de control de dopaje.
3. Cómo usar Fixaprost
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada para adultos (incluyendo pacientes de edad avanzada) es de una gota en el ojo o en los ojos afectados una vez al día.
No utilice Fixaprost más de una vez al día ya que la eficacia del tratamiento puede disminuir si se administra con mayor frecuencia.
Utilice Fixaprost tal y como su médico le ha indicado hasta que le diga que lo suspenda.
Su médico puede querer hacerle pruebas adicionales de corazón y circulatorias si está utilizando Fixaprost.
Usuarios de lentes de contacto
Si usted utiliza lentes de contacto, debe quitárselas antes de utilizar Fixaprost. Después de la aplicación de Fixaprost, debe esperar 15 minutos antes de volver a ponerse las lentes de contacto.
Instrucciones de uso
Este medicamento debe administrarse en el ojo.
Siga estas instrucciones para utilizar el colirio:
- Lávese las manos y siéntase o permanezca cómodamente de pie
- Abra el sobre que contiene 5 envases unidosis. Anote la fecha de primera apertura en el sobre.
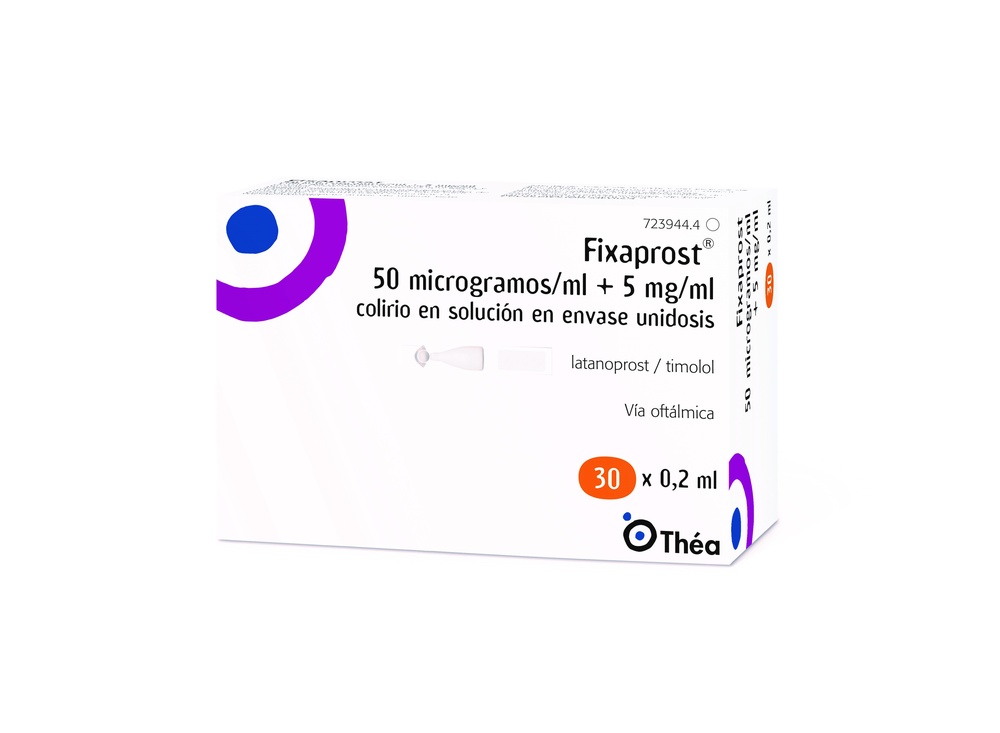
- Separe un envase unidosis de la tira.
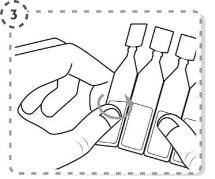
- Gire la punta del envase unidosis tal y como se muestra. No toque la punta después de abrir el envase.
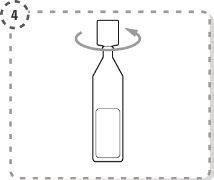
- Utilice su dedo para separar con suavidad el párpado inferior del ojo afectado.
- Coloque la punta del envase unidosis cerca del ojo, pero sin llegar a tocarlo.
- Presione con suavidad el envase unidosis de forma que caiga una gota en el ojo y luego retire el dedo del párpado inferior.
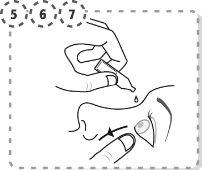
- Presione con el dedo el extremo del ojo afectado, en la parte cercana a la nariz. Ejerza la presión durante 2 minutos, manteniendo su ojo cerrado.
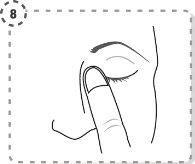
- Repita la operación en el otro ojo, si su médico se lo ha indicado. Cada envase unidosis contiene cantidad suficiente para los dos ojos.
- Deseche el envase unidosis después de utilizarlo. No lo guarde para utilizarlo otra vez. Como no se puede garantizar la esterilidad del envase unidosis tras su apertura, se debe abrir un nuevo envase antes de cada uso.
- Coloque los envases unidosis sin abrir dentro del sobre. Coloque el sobre abierto dentro de la caja. Los envases sin abrir pueden utilizarse en 1 mes tras la apertura del sobre.
Si usa Fixaprost con otros colirios
Espere al menos 5 minutos entre la aplicación de Fixaprost y la administración de otros colirios.
Si usa más Fixaprost del que debe
Si se ha aplicado más gotas en el ojo de las que debía, puede sentir una ligera irritación en el ojo y también puede que los ojos se pongan rojos y que lloren. Esta situación debería desaparecer, pero si le preocupa, contacte con su médico.
Si ingiere Fixaprost
En caso de una ingestión accidental de Fixaprost, póngase en contacto con su médico. Si ingiere una gran cantidad de Fixaprost, puede sentirse mal, tener dolor de estómago, sentirse cansado, acalorado, mareado y empezar a sudar.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20 indicando el medicamento y la cantidad ingerida.
Si olvidó usar Fixaprost
Continúe con la administración de la siguiente dosis de la forma habitual. No utilice una dosis doble para compensar la dosis olvidada. Si tiene dudas consulte con su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Puede seguir utilizando el colirio de forma habitual, a no ser que los efectos adversos sean graves. Si está preocupado, consulte a su médico o farmacéutico. No deje de utilizar Fixaprost sin consultar con su médico.
A continuación se incluyen los efectos adversos conocidos con la utilización de Fixaprost. El efecto adverso más importante es la posibilidad de un cambio gradual y permanente en el color del ojo. Es también posible que Fixaprost pueda causar cambios graves en la forma en que el corazón trabaja. Si usted nota algún cambio en el ritmo cardiaco o en la función cardiaca, debe consultar con un médico y decirle que ha estado utilizando Fixaprost.
Los siguientes efectos adversos pueden presentarse con Fixaprost:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Cambio gradual en el color de los ojos por el incremento de la cantidad de pigmento de color marrón de la parte coloreada del ojo conocida como iris. Si tiene ojos de color mixto (azul-marrón, gris- marrón, amarillo-marrón o verde-marrón) es más probable que sufra este cambio que si sus ojos son de un solo color (azul, gris, verde o marrón). El cambio en el color de los ojos puede tardar años en desarrollarse. El cambio de color puede ser permanente y puede ser más llamativo si utiliza Fixaprost únicamente en un ojo. El cambio en el color del ojo no parece estar asociado a la aparición de ningún problema. El cambio en el color del ojo no progresa una vez que se ha suspendido el tratamiento con Fixaprost.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Irritación del ojo (sensación de escozor, sensación de arenilla en el ojo, picor, pinchazos y sensación de cuerpo extraño en el ojo) y dolor en el ojo.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Dolor de cabeza.
- Enrojecimiento de los ojos, infección del ojo (conjuntivitis), visión borrosa, lagrimeo, inflamación de los párpados, irritación o erosión de la superficie del ojo.
- Sarpullido o picor en la piel (prurito).
- Náuseas, vómitos.
Otrosefectos adversos
Al igual que otros medicamentos utilizados en los ojos, Fixaprost (latanoprost y timolol) se absorbe en la sangre. La incidencia de efectos adversos después de usar colirios es menor que cuando los medicamentos se toman por vía oral o se inyectan.
Aunque no se han visto con Fixaprost, los siguientes efectos adversos se han observado con alguno de los componentes de Fixaprost (latanoprost y timolol) y por lo tanto, podrían producirse con el uso de Fixaprost. Los efectos adversos indicados incluyen reacciones observadas dentro del grupo de los betabloqueantes (por ejemplo, timolol) cuando se utilizan para tratar afecciones del ojo:
- Desarrollo de una infección vírica en el ojo causada por el virus del herpes simple (VHS).
- Reacciones alérgicas generalizadas que incluyen hinchazón bajo la piel que puede ocurrir en zonas como la cara y las extremidades y que pueden obstruir la vía respiratoria causando dificultad para tragar o respirar, urticaria o erupción con picor, erupción localizada o generalizada, picor, reacción alérgica repentina, grave y potencialmente mortal.
- Niveles bajos de glucosa en sangre.
- Mareo.
- Dificultad para dormir (insomnio), depresión, pesadillas, pérdida de memoria, alucinaciones.
- Desmayos, ictus, suministro insuficiente de sangre al cerebro, aumento de los signos y síntomas de miastenia gravis(transtorno muscular), sensación inusual como de agujetas y dolor de cabeza.
- Hinchazón de la parte posterior del ojo (edema macular), quiste lleno de líquido en la parte coloreada del ojo (quiste del iris), sensibilidad a la luz (fotofobia), apariencia de ojos hundidos (mayor profundidad del surco del párpado)
- Signos y síntomas de irritación ocular (por ejemplo, quemazón, pinchazos, picor, lagrimeo, enrojecimiento), inflamación del párpado, inflamación en la córnea, visión borrosa y desprendimiento de la capa que se encuentra bajo la retina y que contiene vasos sanguíneos tras la cirugía de filtración que puede causar alteraciones visuales, sensibilidad corneal disminuida, ojos secos, erosión corneal (daño en la capa anterior del globo ocular), caída del párpado superior (haciendo que el ojo quede medio cerrado), visión doble.
- Oscurecimiento de la piel que rodea a los ojos, cambios en las pestañas y en el vello fino alrededor del ojo (aumento del número, la longitud, el grosor y el oscurecimiento), cambios en la dirección de crecimiento de las pestañas, hinchazón alrededor del ojo, hinchazón de la parte coloreada del ojo (iritis/uveítis), cicatrices en la superficie del ojo.
- Pitidos/zumbidos en los oídos (tinnitus).
- Angina, agravamiento de la angina en pacientes que ya padecían enfermedad del corazón.
- Frecuencia cardiaca baja, dolor de pecho, palpitaciones (sentir el ritmo cardiaco), edema (acumulación de líquido), cambios en el ritmo o velocidad del latido del corazón, insuficiencia cardiaca congestiva (enfermedad del corazón con dificultad en la respiración e hinchazón de pies y piernas por acumulación de líquido), un tipo de trastorno del ritmo del corazón, ataque cardiaco, insuficiencia cardiaca.
- Tensión arterial baja, escasa circulación de la sangre que hace que los dedos de las manos y los pies estén entumecidos y pálidos, manos y pies frios.
- Dificultad en la respiración, constricción de las vías respiratorias en los pulmones (predominantemente en pacientes con enfermedad preexistente), dificultad para respirar, tos, asma, empeoramiento del asma.
- Alteración del gusto, náuseas, indigestión, diarrea, boca seca, dolor abdominal, vómitos.
- Caída del cabello, erupción de la piel con apariencia blanca plateada (erupción psoriasiforme) o empeoramiento de la psoriasis, erupción de la piel.
- Dolor de las articulaciones, dolor muscular que no está causado por el ejercicio, debilidad muscular, cansancio.
- Disfunción sexual, disminución de la líbido.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fixaprost
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja, sobre y envases unidosis. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de temperatura para su conservación.
Tras la primera apertura del sobre:utilizar los envases unidosis en 1 mes.
Escriba la fecha de primera apertura del sobre.
Tras la primera apertura del envase unidosis:utilizar inmediatamente y desechar el envase unidosis después de utilizarlo.
Guarde los envases unidosis sin utilizar dentro del sobre abierto para protegerlos de la luz.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Fixaprost
Los principios activos son latanoprost 50 microgramos/ml y timolol (como timolol maleato) 5 mg/ml.
Los demás componentes son: hidroxiestearato de macrogolglicerol, sorbitol, macrogol, carbómero, edetato de disodio, hidróxido de sodio (para ajustar el pH), agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Este medicamento se presenta como un colirio en solución, en envases unidosis.
La solución es ligeramente amarilla y es una solución opalescente sin conservantes, prácticamente libre de partículas, presentada en el interior de un sobre con 5 unidades, cada envase unidosis contiene 0,2 ml de colirio en solución.
Las cajas contienen 30 (6 x 5) o 90 (18 x 5) envases unidosis.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Laboratoires THEA
12, rue Louis Blériot
63017 CLERMONT-FERRAND Cedex 2
FRANCIA
Fabricante
EXCELVISION
27, rue de la Lombardière
07100 Annonay
FRANCIA
o
Laboratoires THEA
12, rue Louis Blériot
63017 CLERMONT-FERRAND Cedex 2
FRANCIA
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
LABORATORIOS THEA, S.A.
C/ Enric Granados nº 86-88, 2ª planta
08008 Barcelona
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria, Eslovaquia, Francia, Italia, Irlanda, Polonia, Reino Unido, República Checa Fixapost
Alemania, Bélgica, Bulgaria, Chipre, España, Grecia, Holanda, Luxemburgo, Portugal Fixaprost
Dinamarca, Estonia, Finlandia, Islandia, Letonia, Lituania, Noruega, Suecia Fixopost
Croacia, Eslovenia Fixalpost
Rumanía Fixanpost
Portugal Monoprost Duo
Fecha de la última revisión de esteprospecto: Marzo 2023
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Precio medio en farmacia18.81 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FIXAPROST 50 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION EN ENVASES UNIDOSISForma farmacéutica: COLIRIO, 10 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Brill Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml+5mg/mlPrincipio activo: timolol, combinationsFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para FIXAPROST 50 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION EN ENVASES UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FIXAPROST 50 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION EN ENVASES UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















