COSENTYX 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN

How to use COSENTYX 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cosentyx 150mg solution for injection in pre-filled pen
secukinumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
‑ This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Cosentyx and what is it used for
- What you need to know before you use Cosentyx
- How to use Cosentyx
- Possible side effects
- Storing Cosentyx
- Contents of the pack and other information
1. What is Cosentyx and what is it used for
Cosentyx contains the active substance secukinumab. Secukinumab is a monoclonal antibody that belongs to a group of medicines known as “interleukin inhibitors”. It works by neutralizing the activity of a protein called IL-17A, which is found in high levels in diseases such as psoriasis, hidradenitis suppurativa, psoriatic arthritis, and axial spondyloarthritis
Cosentyx is used to treat the following inflammatory diseases:
- Plaque psoriasis
- Hidradenitis suppurativa
- Psoriatic arthritis
- Axial spondyloarthritis, including ankylosing spondylitis (radiographic axial spondyloarthritis) and non-radiographic axial spondyloarthritis
- Juvenile idiopathic arthritis, including enthesitis-related arthritis and juvenile psoriatic arthritis
Plaque Psoriasis
Cosentyx is used to treat a skin condition called “plaque psoriasis” that causes inflammation of the skin. Cosentyx reduces inflammation and other symptoms of the disease. Cosentyx is used in adults, adolescents, and children (from 6 years of age) with moderate to severe plaque psoriasis.
Using Cosentyx for plaque psoriasis will benefit you as it improves the appearance of the skin and reduces symptoms such as scaling, itching, and pain.
Hidradenitis Suppurativa
Cosentyx is used to treat a disease called hidradenitis suppurativa, also known as inverse acne or Verneuil's disease. This is a chronic and painful inflammatory skin disease. Symptoms can include painful nodules (lumps) and abscesses (boils) that can ooze pus. It commonly affects specific areas of the skin, such as under the breasts, armpits, inner thighs, groin, and buttocks. Scarring can also occur in the affected areas.
Cosentyx can reduce the number of nodules and abscesses you have and the pain that is often associated with the disease. If you have hidradenitis suppurativa, you will first be given other medicines. If you do not respond well enough to these medicines, you will be given Cosentyx.
Cosentyx is used in adults with hidradenitis suppurativa and can be used alone or with antibiotics.
Psoriatic Arthritis
Cosentyx is used to treat a condition called “psoriatic arthritis”. This is an inflammatory disease of the joints, often accompanied by psoriasis. If you have active psoriatic arthritis, you will first be given other medicines. If you do not respond well enough to these medicines, you will be given Cosentyx to reduce the signs and symptoms of active psoriatic arthritis, improve physical function, and slow down damage to the cartilage and bones of the joints involved in the disease.
Cosentyx is used in adults with active psoriatic arthritis and can be used alone or with another medicine called methotrexate.
Using Cosentyx for psoriatic arthritis will benefit you as it reduces the signs and symptoms of the disease, slows down damage to the cartilage and bones of the joints, and improves your ability to perform normal daily activities.
Axial Spondyloarthritis, including Ankylosing Spondylitis (Radiographic Axial Spondyloarthritis) and Non-Radiographic Axial Spondyloarthritis
Cosentyx is used to treat conditions called “ankylosing spondylitis” and “non-radiographic axial spondyloarthritis”. These are inflammatory diseases that mainly affect the spine, causing inflammation of the joints of the spine. If you have ankylosing spondylitis or non-radiographic axial spondyloarthritis, you will first be given other medicines. If you do not respond well enough to these medicines, you will be given Cosentyx to reduce the signs and symptoms of the disease, reduce inflammation, and improve physical function.
Cosentyx is used in adults with active ankylosing spondylitis and active non-radiographic axial spondyloarthritis.
Using Cosentyx for ankylosing spondylitis and non-radiographic axial spondyloarthritis will benefit you as it reduces the signs and symptoms of the disease and improves physical function.
Juvenile Idiopathic Arthritis (Enthesitis-Related Arthritis and Juvenile Psoriatic Arthritis)
Cosentyx is used in patients (from 6 years of age) to treat juvenile idiopathic arthritis in the categories called “enthesitis-related arthritis” and “juvenile psoriatic arthritis”. These are inflammatory diseases that affect the joints and the places where tendons join the bone.
Using Cosentyx in enthesitis-related arthritis and juvenile psoriatic arthritis will benefit you (or your child) by reducing symptoms and improving physical function (or your child's physical function).
2. What you need to know before you use Cosentyx
Do not use Cosentyx:
- if you are allergicto secukinumab or any of the other ingredients of this medicine (listed in section 6).
If you think you may be allergic, consult your doctor before using Cosentyx.
- if you have any active infectionthat your doctor thinks is important (for example, active tuberculosis).
Warnings and precautions
Talk to your doctor, nurse or pharmacist before using Cosentyx:
- if you have had an infection.
- if you get repeated or long-lasting infections.
- if you have ever had an allergic reaction to latex.
- if you have a condition that causes inflammation of the gut called Crohn's disease.
- if you have a condition that causes inflammation of the large intestine called ulcerative colitis.
- if you have recently been vaccinated or are about to be vaccinated during treatment with Cosentyx.
- if you are having any other treatment for psoriasis, such as other immunosuppressants or ultraviolet (UV) light therapy.
Tuberculosis
Tell your doctor if you have or have had tuberculosis or if you have recently been in close contact with someone who has tuberculosis. Your doctor will check you for tuberculosis and may do a test for tuberculosis before you start Cosentyx. If your doctor thinks you are at risk of getting tuberculosis, you may be given medicines to treat it. If you get symptoms of tuberculosis (such as a long-lasting cough, weight loss, tiredness or a low-grade fever) while you are being treated with Cosentyx, tell your doctor straight away.
Hepatitis B
Tell your doctor if you have or have had hepatitis B infection. This medicine may cause the infection to come back. Before and during treatment with secukinumab, your doctor may check you for signs of infection. Tell your doctor if you notice any of the following symptoms: worsening tiredness, yellowing of the skin or the white of the eyes, dark urine, loss of appetite, nausea and/or pain in the upper right side of the stomach.
Inflammatory bowel disease (Crohn's disease or ulcerative colitis)
Stop using Cosentyx and tell your doctor or seek medical help straight away if you notice stomach cramps and pain, diarrhea, weight loss, blood in your stools or any other sign of bowel problems.
Watch for signs of infection and allergic reactions
Cosentyx may cause serious side effects, including infections and allergic reactions. You should watch for signs of these conditions while you are using Cosentyx.
Stop using Cosentyx and tell your doctor or seek medical help straight away if you notice any signs of a possible serious infection or allergic reaction. These signs are listed in section 4 “Possible side effects”.
Children and adolescents
Cosentyx is not recommended for use in children under 6 years of age with plaque psoriasis as it has not been studied in this age group.
Cosentyx is not recommended for use in children under 6 years of age with juvenile idiopathic arthritis (enthesitis-related arthritis and juvenile psoriatic arthritis).
Cosentyx is not recommended for use in children and adolescents (under 18 years of age) for other indications as it has not been studied in this age group.
Other medicines and Cosentyx
Tell your doctor or pharmacist:
- if you are taking, have taken or might take any other medicines.
- if you have recently been vaccinated or are about to be vaccinated. You should not be given certain types of vaccines (live vaccines) while you are using Cosentyx.
Pregnancy, breastfeeding and fertility
- It is recommended that you avoid using Cosentyx during pregnancy. The effect of this medicine in pregnant women is not known. If you are a woman who can become pregnant, you are advised to avoid becoming pregnant and to use a suitable contraceptive while you are using Cosentyx and for at least 20 weeks after the last dose of Cosentyx. If you are pregnant or think you may be pregnant, consult your doctor.
- Ask your doctor if you are breastfeeding or plan to breastfeed. You and your doctor must decide whether you will breastfeed or use Cosentyx. You must not do both. After using Cosentyx, you must not breastfeed for at least 20 weeks after the last dose.
Driving and using machines
Cosentyx is unlikely to affect your ability to drive or use machines.
3. How to use Cosentyx
Follow the instructions for administration of this medicine exactly as your doctor has told you. If you are not sure, ask your doctor, pharmacist or nurse.
Cosentyx is given by injection under the skin (i.e. subcutaneously). You and your doctor will decide if you should inject Cosentyx yourself.
Do not try to inject Cosentyx until you have been trained by your doctor, nurse or pharmacist. The person who cares for you can also give you the injection of Cosentyx after they have received training.
Instructions on how to inject Cosentyx are given in the section “Instructions for use of Cosentyx 150 mg in pre-filled pen” at the end of this leaflet.
Instructions for use can also be found via the following QR code and website:
‘QR code to be included’
www.cosentyx.eu
How much Cosentyx to use and for how long
Your doctor will decide how much Cosentyx you need and for how long.
Plaque Psoriasis
Adults
- The recommended dose is 300 mg given as a subcutaneous injection.
- A dose of 300 mg is given as two injections of 150mg.
After the first dose, you will receive injections every week in weeks 1, 2, 3 and 4 followed by monthly injections. Depending on your response, your doctor may recommend additional dose adjustments. At each visit, you will receive a dose of 300 mg given as two injections of 150 mg.
Children from 6 years of age
- The recommended dose is based on body weight as follows:
- Weight below 25 kg: 75 mg given as a subcutaneous injection.
- Weight from 25 kg to below 50 kg: 75 mg given as a subcutaneous injection.
- Weight from 50 kg: 150 mg given as a subcutaneous injection.
Your doctor may increase the dose to 300 mg.
- Each dose of 150 mg is given as one injection of 150mg. Other formulations/concentrations may be available for administration of 75 mg and 300 mg doses.
After the first dose, you will receive injections every week in weeks 1, 2, 3 and 4 followed by monthly injections.
Hidradenitis Suppurativa
- The recommended dose is 300 mg given as a subcutaneous injection.
- Each dose of 300 mg is given as two injections of 150mg.
After the first dose, you will receive injections every week in weeks 1, 2, 3 and 4 followed by monthly injections. Depending on your response, your doctor may recommend additional dose adjustments.
Psoriatic Arthritis
If you have psoriatic arthritis and also have moderate to severe plaque psoriasis, your doctor may adjust the recommended dose as needed.
For patients who have not responded well to medicines known as tumor necrosis factor (TNF) blockers:
- The recommended dose is 300 mg given as a subcutaneous injection.
- Each dose of 300 mg is given as two injections of 150mg.
After the first dose, you will receive injections every week in weeks 1, 2, 3 and 4 followed by monthly injections. At each visit, you will receive a dose of 300 mg given as two injections of 150 mg.
For other patients with psoriatic arthritis:
- The recommended dose is 150 mg given as a subcutaneous injection.
After the first dose, you will receive injections every week in weeks 1, 2, 3 and 4 followed by monthly injections.
Your doctor may increase your dose to 300 mg.
Ankylosing Spondylitis (Radiographic Axial Spondyloarthritis)
- The recommended dose is 150 mg given as a subcutaneous injection.
After the first dose, you will receive injections every week in weeks 1, 2, 3 and 4 followed by monthly injections.
Your doctor may increase your dose to 300 mg. Each dose of 300 mg is given as two injections of 150 mg.
Non-Radiographic Axial Spondyloarthritis
- The recommended dose is 150 mg given as a subcutaneous injection.
After the first dose, you will receive injections every week in weeks 1, 2, 3 and 4 followed by monthly injections.
Juvenile Idiopathic Arthritis (Enthesitis-Related Arthritis and Juvenile Psoriatic Arthritis)
- The recommended dose is based on body weight as follows:
- Weight below 50 kg: 75 mg given as a subcutaneous injection.
- Weight from 50 kg: 150 mg given as a subcutaneous injection.
- Each dose of 150 mg is given as one injection of 150mg. Other formulations/concentrations may be available for administration of the 75 mg dose.
After the first dose, you (or your child) will receive injections every week in weeks 1, 2, 3 and 4 followed by monthly injections.
Cosentyx is a long-term treatment. Your doctor will regularly check the state of your disease to see if the treatment is working.
If you use more Cosentyx than you should
If you receive more Cosentyx than you should or the dose has been given before the time indicated by your doctor, tell your doctor.
If you forget to use Cosentyx
If you have forgotten to inject a dose of Cosentyx, inject the next dose as soon as you remember. Then talk to your doctor to find out when you should inject the next dose.
If you stop using Cosentyx
It is not dangerous to stop using Cosentyx. However, if you do, it is possible that the symptoms of psoriasis, psoriatic arthritis or axial spondyloarthritis may come back.
If you have any other questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Severe Adverse Effects
Discontinue treatment with Cosentyx and inform your doctor or seek immediate medical attention if you notice any of the following adverse effects:
Possible Severe Infection– signs may include:
- fever, flu-like symptoms, night sweats
- feeling of fatigue or difficulty breathing, persistent cough
- hot, red, and painful skin to the touch, or painful rash with blisters
- burning when urinating.
Severe Allergic Reaction– signs may include:
- difficulty breathing or swallowing
- low blood pressure, which can cause dizziness or slight dizziness
- swelling of the face, lips, tongue, or throat
- intense itching of the skin accompanied by rash or blisters.
Your doctor will decide if you should and when to restart treatment.
Other Adverse Effects
Most of the following adverse effects are mild or moderate. If they become severe in any case, inform your doctor, pharmacist, or nurse.
Very Common(may affect more than 1 in 10 people):
- upper respiratory tract infections with symptoms such as sore throat and nasal congestion (rhinopharyngitis, rhinitis).
Common(may affect up to 1 in 10 people):
- mouth ulcers (oral herpes)
- diarrhea
- nasal discharge (rhinorrhea)
- headache
- nausea
- fatigue
- itching, redness, and dryness of the skin (eczema)
Uncommon(may affect up to 1 in 100 people):
- oral thrush (oral candidiasis)
- signs of white blood cell deficiency, such as fever, sore throat, or mouth ulcers due to infections (neutropenia)
- outer ear infection (otitis externa)
- eye discharge with itching, redness, and swelling (conjunctivitis)
- itchy rash (urticaria)
- lower respiratory tract infections
- abdominal cramps, abdominal pain, diarrhea, weight loss, or blood in the stool (signs of intestinal problems)
- small, itchy blisters on the palms of the hands, soles of the feet, and edges of the fingers and toes (dyshidrotic eczema)
- athlete's foot (tinea pedis)
Rare(may affect up to 1 in 1,000 people):
- severe allergic reaction with shock (anaphylactic reaction)
- redness and peeling of the skin over a large area of the body, which can be itchy or painful (exfoliative dermatitis)
- inflammation of small blood vessels, which can lead to a skin rash with small red or purple bumps (vasculitis)
- swelling of the neck, face, mouth, or throat that can lead to difficulty swallowing or breathing (angioedema)
Frequency Not Known(cannot be estimated from available data):
- fungal infections of the skin and mucous membranes (including esophageal candidiasis)
- painful swelling and ulceration of the skin (pyoderma gangrenosum)
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Cosentyx
Keep this medicine out of sight and reach of children.
Do not use this medicine:
- after the expiration date that appears on the box or on the label of the pen after "EXP".
Store the sealed pen in its box to protect it from light. Store in the refrigerator between 2°C and 8°C. Do not freeze. Do not shake.
If necessary, Cosentyx can be left out of the refrigerator for a single period of up to 4 days at room temperature, not exceeding 30°C.
This medicine is for single use only.
Medicines should not be thrown down the drain. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Cosentyx Composition
- The active ingredient is secukinumab. Each preloaded pen contains 150 mg of secukinumab.
- The other components are trehalose dihydrate, histidine, histidine hydrochloride monohydrate, methionine, polysorbate 80, and water for injectable preparations.
Appearance of Cosentyx and Package Contents
Cosentyx injectable solution is a clear liquid. Its color may vary from colorless to slightly yellow.
Cosentyx 150 mg injectable solution in preloaded pen is presented in unit packs of 1 or 2 preloaded pens and in multiple packs containing 6 (3 packs of 2) preloaded pens.
Not all pack sizes may be marketed.
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Pharma GmbH
Roonstraße 25
90429 Nuremberg
Germany
Sandoz GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
Novartis Pharmaceutical Manufacturing GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lithuania SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estonia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norway Novartis Norge AS Tel: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Poland Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma – Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croatia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Tel: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia SIA Novartis Baltics Tel: +371 67 887 070 |
Date of Last Revision of this Prospectus:11/2024
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
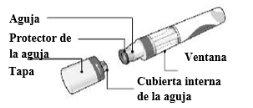
Instructions for Use of Cosentyx 150 mg in SensoReady Pen
| Cosentyx 150 mgin SensoReady Pen Injectable solution in preloaded pen Secukinumab Patient Instructions for Use |
| READ ALL INSTRUCTIONS BEFORE INJECTING THE MEDICINE. These instructions will help you correctly administer Cosentyx in the SensoReady pen. It is essential that you do not attempt to inject the medicine or a person under your care until the doctor, nurse, or pharmacist has taught you how to do it. |
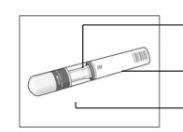
Your Cosentyx 150 mg in SensoReady Pen:
Cosentyx 150 mg in SensoReady Pen without cap. Do notremove the cap until you are ready to administer the injection. | Keep the box with the pen in the refrigeratorbetween 2°C and 8°C and out of the reach of children.
For a more comfortable injection, remove the pen from the refrigerator 15-30 minutesbefore to allow it to reach room temperature. |
What you need for the injection:
Included in the box: A new, unused Cosentyx 150 mg SensoReady pen (1 pen is needed for a 150 mg dose and 2 pens for a 300 mg dose).
| Not included in the box:
|
|
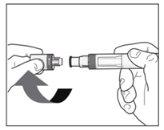
Before the injection:
|
The liquid should be transparent. Its color may vary from colorless to slightly yellow. |
Do not useif the liquid contains particles, is cloudy, or has a clearly brown color. You may see small air bubbles, which is normal. | |
Do not usethe pen if it has expired. | |
Do not useif the sealis broken. Contact your pharmacist if the pen does not meet any of these requirements. | |
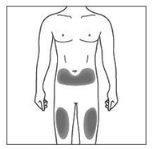
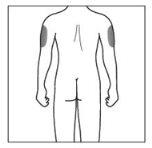
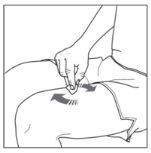
| 2a. Choose an injection site:
|
| 2b. Only caregivers or healthcare professionals:
|
|
|
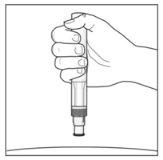
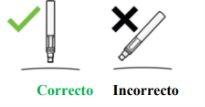
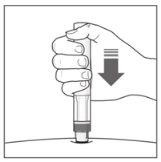
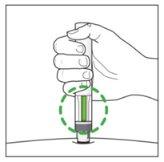
The Injection:
|
|
|
|
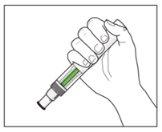
| YOU MUST READ THE FOLLOWING BEFORE INJECTION. During the injection, you will hear 2loud clicks. The 1st clickindicates the start of the injection. After a few seconds, the 2nd clickwill indicate that the injection is about to finish. Keep the pen firmly pressed against the skin until the green indicatorfills the window and stops moving. |
|
|
|
|
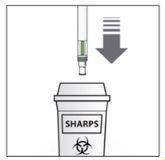
After the injection:
|
|
|
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COSENTYX 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PENDosage form: INJECTABLE, 150mg/1mlActive substance: secukinumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 150 mg secukinumab / mlActive substance: secukinumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 150mg/1mlActive substance: secukinumabManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for COSENTYX 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Discuss questions about COSENTYX 150 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions