CLARELUX 500 micrograms/g CUTANEOUS FOAM IN A PRESSURIZED CONTAINER


How to use CLARELUX 500 micrograms/g CUTANEOUS FOAM IN A PRESSURIZED CONTAINER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
CLARELUX 500 micrograms/g cutaneous foam in a pressurized container
Clobetasol propionate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is CLARELUX and what is it used for
- What you need to know before you use CLARELUX
- How to use CLARELUX
- Possible side effects
- Storing CLARELUX
- Contents of the pack and other information
1. What is CLARELUX and what is it used for
CLARELUX contains clobetasol propionate as the active ingredient and belongs to a group of medicines known as topical corticosteroids. Clobetasol propionate is a very potent topical corticosteroid.
CLARELUX 500 micrograms/g cutaneous foam in a pressurized container is a foam that is applied to the skin.
CLARELUX 500 micrograms/g cutaneous foam in a pressurized container is used, in adult and adolescent patients from 12 years of age, as a short-term treatment for scalp dermatoses that respond to steroids, such as: psoriasis, which does not respond satisfactorily to less potent steroids.
2. What you need to know before you use CLARELUX
Do not useCLARELUX:
- If you are allergic to clobetasol propionate, other corticosteroids or any of the other ingredients of this medicine (listed in section 6).
- If you have infected skin lesions, whether viral (e.g. herpes simplex, herpes zoster, chickenpox...), bacterial (e.g. impetigo), fungal (caused by microscopic fungi) or parasitic.
- If you have burns, ulcerative lesions or other skin disorders such as rosacea, acne, dermatitis around the mouth.
- If you have itching near the anus or genitals.
- On another area of the body or face (including eyelids) that is not the scalp.
- On the eyelids (risk of damage to the eye nerve (glaucoma) and lens opacity (cataracts)).
- In children under 2 years of age.
Warnings and precautions
Consult your doctor or pharmacist before starting to use CLARELUX.
Tell your doctor if you have previously had an allergy to corticosteroids and/or to any component of this medicine.
You should consult with your doctor and interrupt treatment immediately if your condition worsens during use, you may be experiencing an allergic reaction, if you present signs such as skin rash, itching or painless swelling of the tissue (edema), if you have an infection or if your condition requires different treatment.
You should avoid long-term treatment.
If you experience a recurrence of your condition soon after (within 2 weeks) of stopping treatment, do not restart using CLARELUX without consulting your doctor, unless your doctor has previously advised you to do so. If your condition has resolved and, in case of recurrence, the redness extends beyond the initial treatment area and you experience a burning sensation, consult a doctor before restarting treatment, as a rebound effect may be suspected (see section 4).
Due to the deterioration of the skin barrier, there is a risk of sudden appearance of painful, non-infectious, fluid-filled blisters that may be accompanied by fever (generalized pustular psoriasis) or local or systemic toxicity.
Avoid contact with the eyes or mucous membranes (nose, mouth).
Do not apply CLARELUX to the eyelids or face due to the risk of lens opacity (cataracts) and increased eye pressure (glaucoma), which can cause irreversible damage to the eyes. Consult your doctor if you experience blurred vision or other visual disturbances.
Wash your hands carefully after each application. Do not touch your eyes until you have washed your hands.
In case of accidental contact with the face or eyes, wash with plenty of water.
Unless supervised by a doctor, application of CLARELUX over a large surface area or on bandaged or covered areas should be avoided, due to the risk of the active ingredient passing into the bloodstream. A bacterial infection may occur, facilitated by the heat and humidity of the skin in occlusive dressings. Do not use an occlusive dressing unless indicated by your doctor. In this case, the skin should be cleaned before each dressing change.
Inform your doctor about any irritation or infection, as appropriate treatment should be administered if an infection occurs. If the infection spreads, treatment with CLARELUX should be discontinued and treated.
As with all corticosteroids, CLARELUX may be absorbed through the skin with a risk of the active ingredient passing into the blood, causing side effects such as decreased production of hormones from the adrenal glands (suppression of the pituitary-adrenal system) and Cushing's syndrome (see section 4 for all possible side effects). The risk of this corticosteroid passing into the bloodstream increases in the following situations:
- Prolonged treatment.
- Application to very large areas.
- Application to bandaged or covered areas, such as occlusive dressings.
- Application to damaged or injured skin, such as open wounds or ulcers.
- Application to areas of thin skin such as the face.
- Greater hydration of the skin.
Tell your doctor if:
- You experience new bone pain or worsening of previous bone symptoms during treatment with CLARELUX, especially if you have been using CLARELUX for a prolonged period or repeatedly.
- You are using another oral or topical medication that contains corticosteroids or medications to control your immune system (e.g. autoimmune disease or after a transplant). The combination of CLARELUX with these medications may result in serious infections.
- Your condition does not improve after 2 weeks of treatment.
- You develop an infection, as treatment with CLARELUX should be discontinued and appropriate antimicrobial therapy administered.
Use in children and adolescents
It is not recommended for use in children under 12 years of age.
Other medicines and CLARELUX
Tell your doctor or pharmacist if you are using or have recently used or may need to use any other medicines.
Using CLARELUX with food, drinks, and alcohol
Not applicable
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
CLARELUX should not be used during pregnancy unless your doctor indicates it.
Breastfeeding
CLARELUX should not be used during breastfeeding unless your doctor indicates it.
Driving and using machines
CLARELUX should not affect your ability to drive and use machines.
Important information about some of the ingredients of CLARELUX
This medicine contains:
- 2145 mg of alcohol (ethanol) in each application, may cause a burning sensation on damaged skin,
- 74 mg of propylene glycol (E 1520) in each application,
- cetostearyl alcohol and stearyl alcohol, which may cause local skin reactions (such as contact dermatitis),
- polysorbate 60 (E 435) may cause allergic reactions.
3. How to use CLARELUX
WARNING:
The container contains aflammable pressurized liquid.
Do not use or store near a flame, ignition source, heat-generating material, or operating electrical products.
Do not smoke while using or holding the container.
Follow your doctor's instructions for using CLARELUX exactly. Consult your doctor or pharmacist if you have any doubts.
Use this medication only for the condition for which it was prescribed. CLARELUX should only be applied to the scalp and should not be swallowed.
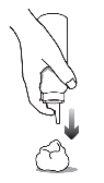
It is not recommended to dispense the foam directly into the hands, as the foam will start to dissolve immediately upon contact with warm skin.
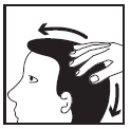
Apply CLARELUX to the affected area of the scalp twice a day, once in the morning and once at night, as follows:
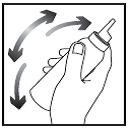
Warning: for proper dispensing of the foam, it is essential to keep the container upside down!
|
|
CLARELUX should be applied in a thin layer, so you should use the minimum amount when applying it to the affected area. The exact amount you need depends on the size of the affected area. Do not dispense the foam into the hands, as it will start to dissolve immediately upon contact with warm skin. |
|
|
|
After applying CLARELUX, wash your hands and remove any unused medication.
Do not use CLARELUX on the face or eyelids. If foam accidentally gets into your eyes, nose, or mouth, rinse immediately with cold water. You will experience a burning sensation. Contact your doctor if the pain continues.
The treated areas should not be bandaged or covered unless indicated by your doctor.
Do not wash or rinse the treated areas of the scalp immediately after applying CLARELUX.
Treatment duration
Do not use more than 50 g of CLARELUX per week.
Treatment should not continue for more than 2 weeks. After this use, CLARELUX may be used occasionally if necessary. Alternatively, your doctor may prescribe a weaker steroid to control your condition.
If you use moreCLARELUXthan you should
Consult your doctor immediately if you have applied CLARELUX:
in quantities greater than prescribed or for a longer period than prescribed. In these cases, there is a risk that the active ingredient will pass into the blood, causing side effects such as symptoms of hypercorticoidism (weight gain, accumulation of fat in the face, and hypertension). The use of CLARELUX should be gradually discontinued under medical supervision, reducing the frequency of application or replacing it with a less potent corticosteroid.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used.
If you forget to useCLARELUX
It should be used as soon as possible, then continue as before.
If you remember when applying the next dose, use a single dose and continue treatment as your doctor has described (do not apply a double dose to make up for forgotten doses). If you have forgotten several doses, inform your doctor.
If you stop treatment withCLARELUX
Do not stop treatment suddenly, as it may cause you harm. Your doctor should discontinue treatment gradually and regular check-ups should be performed.
If you have any other questions about using this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using CLARELUX 500 micrograms/g cutaneous foam in a pressurized container and contact your doctor immediately if you experience allergic reactions (hypersensitivity), such as local irritation.
Side effects include:
Common (may affect up to 1 in 10 people but more than 1 in 100 people):
- Skin burning sensation where CLARELUX is applied
- Other skin reactions where CLARELUX is applied
Uncommon (may affect up to 1 in 1,000 people):
- Pustular psoriasis (chronic skin inflammation accompanied by pustules)
Rare (may affect up to 1 in 10,000 people):
- Decreased production of hormones from the adrenal glands (suppression of the pituitary-adrenal system)
- Numbness, tingling, or pinching sensation (paresthesia)
- Eye irritation
- Vasodilation
- Skin irritation, skin pain (sensitivity), skin tightness
- Contact dermatitis, skin inflammation (dermatitis)
- Worsening of psoriasis
- Redness (erythema) at the application site
- Itching (pruritus) at the application site
- Pain
- Presence of blood, protein, and nitrogen in urine
- Abnormal blood test results indicating that red blood cells are larger than average (increased mean corpuscular volume)
Frequency not known (cannot be estimated from the available data):
- A secondary infection may occur, especially if the treatment is covered with an occlusive dressing or applied to skin folds (armpits, anal and genital area). Signs of infection include skin redness, possibly accompanied by pain or itching.
- Excessive hair growth (hypertrichosis)
- Changes in skin color
- Inflammation of the hair follicles (folliculitis)
- Mouth rash (perioral dermatitis)
- Redness and rash on the face (rosacea-like dermatitis)
- Delayed wound healing
- Lens opacity (cataracts), increased eye pressure (glaucoma)
- Blurred vision
Side effects due to prolonged use with frequency not known (cannot be estimated from the available data):
- As with other topical corticosteroids, when CLARELUX is used in large quantities and for a long period, it may lead to Cushing's syndrome, which includes signs such as weight gain, accumulation of fat in the face, and bruising caused by an excess of corticosteroid hormone.
- Topical corticosteroid withdrawal reaction (rebound effect): skin redness that may extend beyond the initially treated area, burning sensation, intense itching, skin peeling, suppurating open sores.
- Local skin changes such as thinning (skin atrophy) and fragility, bruising (ecchymosis), visible small blood vessels (telangiectasias) especially on the face, stretch marks that affect the proximal limbs in particular.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing CLARELUX
- The container contains aflammable pressurized liquid.
- Do not store near a flame, ignition source, heat-generating material, or operating electrical products.
- Do not expose to temperatures above50°Cor direct sunlight.
- Do not puncture or burn the container, even if it is empty.
- When treatment is finished,dispose of the container safely.
Keep this medicine out of the sight and reach of children.
Do not use CLARELUX after the expiration date that appears on the container after EXP. The expiration date is the last day of the month indicated.
Do not store above 25°C. Do not refrigerate. Store upright.
Medicines should not be disposed of via wastewater or household waste. Deposit the containers and medicines you no longer need at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of containers and medicines you no longer need. This will help protect the environment.
6. Container contents and additional information
Composition ofCLARELUX
The active ingredient is clobetasol propionate and 1 g of skin foam contains 500 micrograms of clobetasol propionate.
The other components are: anhydrous ethanol, purified water, propylene glycol (E 1520), cetyl alcohol, stearyl alcohol, polysorbate 60 (E 435), anhydrous citric acid, potassium citrate, and a propellant mixture of propane/n-butane/isobutane.
Appearance of the product and container contents
CLARELUX 500 micrograms/g skin foam is a white skin foam in a pressurized container. Each container contains 50 or 100 grams.
Not all containers may be marketed.
Marketing authorization holder
PIERRE FABRE IBÉRICA, S.A.
C/ Ramón Trias Fargas, 7-11
08005 Barcelona - Spain
Manufacturer
Recipharm Uppsala AB
Björkgatan 30
751 82 Uppsala
Sweden
or
Farmol Health Care S.r.L.
Via del Maglio, 6
23868 Valmadrera (LC)
Italy
This medicinal product is authorized in the Member States of the European Economic Areaand in the United Kingdom (Northern Ireland)under the following names:
Germany and Austria:CLARELUX 500 Mikrogramm/g Schaum zur Anwendung auf der Haut im Druckbehältnis
Belgium and Luxembourg:CLARELUX 500 microgrammes/g mousse pour application cutanée en flacon pressurisé
Slovak Republic:CLARELUX 500 mikrogramov/g dermalna pena, v tlakovom obale
Czech Republic:CLARELUX 500 mikrogramu/g kožní pena
France:CLARELUX 500 microgrammes/g mousse pour application cutanée en flacon pressurisé
Greece:CLARELUX 500 μικρογραμμ?ρια/g δερματικ?ς αφρ?ς σε περι?κτη υπ? π?εση
United Kingdom (Northern Ireland):CLARELUX 500 microgram/g cutaneous foam in pressurised container
Netherlands:CLARELUX 500 microgram/g schuim voor cutaan gebruik in spuitbus
Poland:Clarelux 500 mikrogramów/g, piana na skóre
Portugal:CLARELUX 500 microgramas/g espuma cutânea num recipiente pressurizado
Italy:OLUX 500 microgrammi/g schiuma cutanea
Date of last revision of this leaflet: May 2025
“Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) https://www.aemps.gob.es/”
- Country of registration
- Average pharmacy price8.7 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CLARELUX 500 micrograms/g CUTANEOUS FOAM IN A PRESSURIZED CONTAINERDosage form: SHAMPOO, 500 mcg clobetasol propionate/gActive substance: clobetasolManufacturer: Laboratorios Galderma S.A.Prescription requiredDosage form: TOPICAL SOLUTION, 500 micrograms/gActive substance: clobetasolManufacturer: Isdin S.A.Prescription requiredDosage form: SHAMPOO, 500 µg/gActive substance: clobetasolManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for CLARELUX 500 micrograms/g CUTANEOUS FOAM IN A PRESSURIZED CONTAINER
Discuss questions about CLARELUX 500 micrograms/g CUTANEOUS FOAM IN A PRESSURIZED CONTAINER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions