
CABLIVI 10 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION


How to use CABLIVI 10 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cablivi 10 mg powder and solvent for solution for injection
caplacizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Cablivi and what is it used for
- What you need to know before you use Cablivi
- How to use Cablivi
- Possible side effects
- Storing Cablivi
- Contents of the pack and other information
1. What is Cablivi and what is it used for
Cablivi contains the active substance caplacizumab. It is used to treat an episode of acquired thrombotic thrombocytopenic purpurain adults and adolescents from 12 years of age who weigh at least 40 kg. This is a rare blood clotting disorder in which clots form in small blood vessels. These clots can block the blood vessels and damage the brain, heart, kidneys, or other organs. Cablivi prevents the formation of these blood clots by preventing the blood platelets from clumping together. In this way, Cablivi reduces the risk of suffering another episode of acquired thrombotic thrombocytopenic purpura (aTTP) soon after the first one.
2. What you need to know before you use Cablivi
Do not use Cablivi
- if you are allergic to caplacizumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Tell your doctor if:
- you bleed excessively or experience unusual symptoms such as headache, difficulty breathing, tiredness, or fainting that may indicate severe internal bleeding. Your doctor may ask you to stop treatment. The doctor will tell you when you can start your treatment again.
- you are using medicines that prevent or treat blood clots such as warfarin, heparin, rivaroxaban, apixaban. Your doctor will decide how you should be treated.
- you are using antiplatelet agents such as aspirin or low molecular weight heparin (which prevent blood clots). Your doctor will decide how you should be treated.
- you have a bleeding disorder, such as hemophilia. Your doctor will decide how you should be treated.
- you have severely impaired liver function. Your doctor will decide how you should be treated.
- you are going to have surgery or dental treatment. Your doctor will decide whether it can be postponed or whether Cablivi should be stopped before surgery or dental treatment.
Children and adolescents
Cablivi is not recommended in children under 12 years of age and weighing less than 40 kg.
Other medicines and Cablivi
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Also, tell your doctor if you are using anticoagulant medicines such as vitamin K antagonists, rivaroxaban, or apixaban to treat blood clots, or antiplatelet agents such as aspirin or low molecular weight heparin, which prevent blood clots.
Pregnancy and breastfeeding
Tell your doctor if you are pregnant or plan to become pregnant. Cablivi is not recommended during pregnancy.
Tell your doctor if you are breastfeeding. Your doctor will advise you whether you should stop breastfeeding or not use Cablivi, taking into account the benefit of breastfeeding for the baby and the benefit of Cablivi for you.
Driving and using machines
Cablivi is not expected to affect your ability to drive or use machines.
Cablivi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially “sodium-free”.
3. How to use Cablivi
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Treatment with Cablivi is started by a doctor with experience in blood disorders.
Treatmentis recommended as follows
- first dose
- 1 vial injected into a vein by a healthcare professional
- the medicine will be administered before starting plasma exchange.
- subsequent doses
- 1 vial once a day as a subcutaneous injection (under the skin of the abdomen)
- the subcutaneous injection will be administered after each daily plasma exchange
- after daily plasma exchange is completed, treatment with Cablivi will continue for at least 30 days with one vial injected once a day
- your doctor may ask you to continue daily treatment until the underlying signs of your disease have resolved.
Your doctor may decide that you or your caregiver will administer the injection of Cablivi. In this case, your doctor or healthcare professional will train you or your caregiver on how to use Cablivi.
Instructions for use
The first injection of Cablivi into your vein must be administered by a healthcare professional. The instructions for healthcare professionals on how to inject Cablivi into the vein are at the end of the leaflet.
For each injection, use a new container to prepare the injection solution. Do not attempt to administer the injection of Cablivi until a healthcare professional has shown you how to do it. Never use the contents of the container for another injection.
Step 1- Cleaning
- Wash your hands well with water and soap.
- Prepare a clean and flat surface to place the contents of the container.
- Make sure you have a container for waste at hand.
Step 2 -Before use
- Make sure the container is complete.
- Check the expiration date. Do not use it after the expiration date.
- Do not use the container if the components it contains are damaged in any way.
- Place all the components of the container on the clean and flat surface.
- If the container was not stored at room temperature, wait for the vial and syringe to reach room temperature (15 °C – 25 °C) by leaving them at room temperature for a few minutes. Do not heat them in any other way.
Step 3- Disinfection of the rubber stopper
- Remove the plastic flip-top cap from the vial. Do not use the vial if the green plastic cap is missing.
- Clean the exposed rubber stopper with one of the provided alcohol wipes and let it dry for a few seconds.
- After cleaning, do not touch the rubber stopper or let it touch any surface.
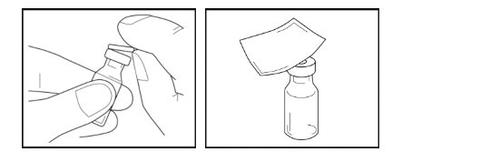
Step 4- Placement of the adapter
- Take the vial adapter from the packaging and remove the paper cover. Leave the adapter in the open plastic container. Do not touch the adapter.
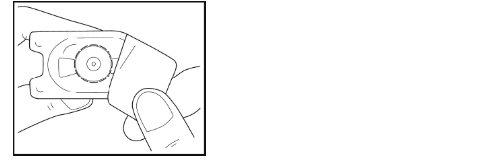
- Place the adapter over the vial, while keeping the adapter in its plastic container.
- Press firmly downwards until the adapter is in place, with the tip of the adapter piercing the vial stopper. Leave the adapter connected to the vial, stillin its outer container.
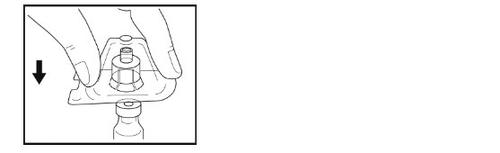
Step 5- Preparation of the syringe
- Holding the syringe in your hand, break the white cap with your other hand.
- Do not use the syringe if the white cap is missing, loose, or damaged.
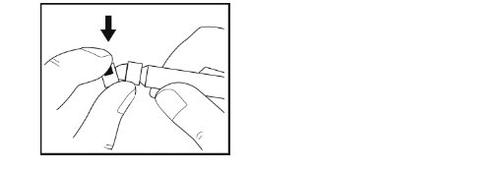
- Do not touchthe tip of the syringe or let it touch any surface.
- Place the syringe on the clean and flat surface.
Step 6– Connection of the syringe to the adapter and vial
- Take the vial with the adapter connected.
- Remove the plastic container from the adapter by holding the vial with one hand, pressing the sides of the adapter container with the other hand, and then lifting the container upwards. Be careful not to dislodge the adapter from the vial.
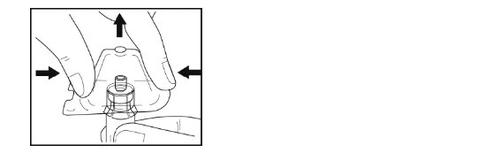
- Holding the adapter with the vial connected with one hand, place the tip of the syringe into the connector part of the vial adapter.
- Gently secure the syringe to the vial by turning it clockwise until you feel resistance.
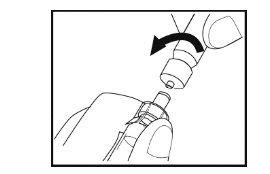
Step 7- Preparation of the solution
- Hold the vial vertically on the surface with the syringe facing downwards.
- Slowly push the syringe plunger downwards until the syringe is empty. Do not remove the syringe from the vial.
- With the syringe still connected to the adapter of the vial, gently turn the vial with the syringe connected until the powder is dissolved. Avoid foaming. Do not shakethe vial.
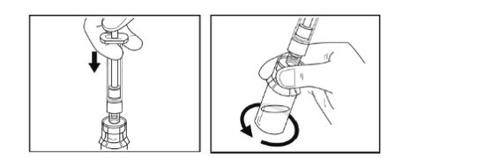
- Let the vial with the syringe connected stand on the surface for 5 minutesat room temperature to allow the solution to dissolve completely. The plunger may rise back up by itself; this is normal.
- Go to step 8 immediately after these 5 minutes.
Step 8- Withdrawal of the solution
- Check if the solutionhas particles. All the powder should have dissolved and the solution should be clear.
- Slowly press the syringe plunger all the way down.
- Turn the vial, adapter, and syringe completely upside down.
- While holding it vertically, slowly pull the plunger to put all the solution into the syringe. Do not shake.
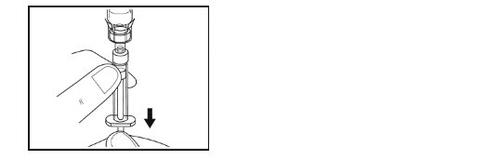
Step 9 – Preparation of the syringe for administration
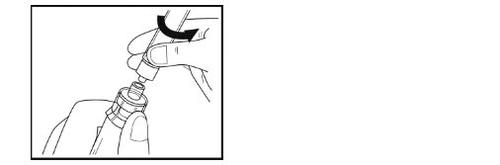
- Turn the vial, adapter, and syringe completely upside down (with the syringe on top). Disconnect the syringe from the adapter by holding the adapter with one hand and gently turning the syringe counterclockwise.
- Place the vial and connected adapter in the provided waste container.
- Do not touchthe tip of the needle or let it touch any surface. Place the syringe on the clean and flat surface.
- Go to step 10 to inject caplacizumab under the skin of the abdomen. The instructions for healthcare professionals on how to inject Cablivi into the vein are at the end of the leaflet.
Step 10 -Insertion of the needle
- Remove the needle from its packaging by breaking the paper cover and removing the needle with the protective cap.
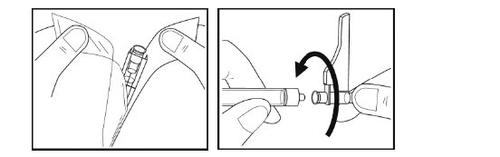
- Without removing the needle cap, insert the needle into the syringe by turning it clockwise until you feel resistance.
- Pull back the needle safety protector.
- Check the contents of the syringe. Do not use the medicine if you notice it is cloudy, has lumps, or anything else that does not seem normal. Contact your doctor or nursing staff if this happens.
Step 11- Preparation of the injection site for subcutaneous injection
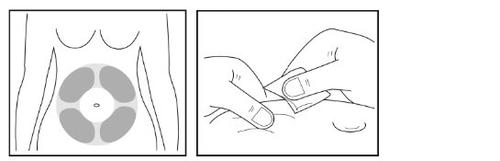
- Select a suitable site (“injection site”) on your abdomen for the subcutaneous injection.
Avoid the area around the navel. Choose a different injection site from the one you used the day before so that the skin can recover after the injection.
- Use the second alcohol wipe to clean the injection site you have chosen.
Step 12- Administration
- Carefully remove the needle protective cap and discard it. Make sure the needle does not touch anything before the injection.
- Hold the syringe at eye level with the needle pointing upwards.
- Eliminate air bubbles by tapping the side of the syringe with your finger so that the bubbles rise to the tip. Then, slowly push the plunger until a small amount of liquid comes out of the needle.
- Gently pinch the clean skin between your thumb and index finger to make a fold.
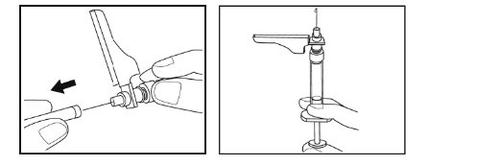
- Maintain this skin fold throughout the injection.
- Insert the entire needle into the skin fold at the angle shown in the illustration.
- Press the plunger downwards as far as it will go.

- Remove the needle with the same angle as you inserted it. Do not rub the injection site.
Step 13- After administration
- Immediately after the injection, place the needle safety protector over the needle until it clicks into place.
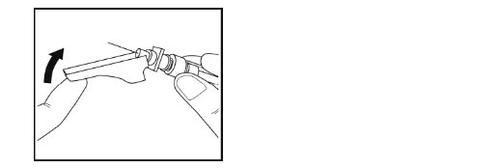
- Put the syringe with the needle in a waste container.
If you use more Cablivi than you should
An overdose is unlikely because a vial contains only a single dose. Inform your doctor if you think you have had an overdose.
If you forget to use Cablivi
If you miss a dose, you should administer it if it has been no more than 12 hours since the scheduled time. If more than 12 hours have passed since the dose was due, do not administer the missed dose, but inject the next dose at the usual time.
If you stop treatment with Cablivi
To get the most benefit from your treatment, it is important to use Cablivi as prescribed and for the duration your doctor indicates. Inform your doctor before stopping treatment, as stopping it too soon may cause your disease to recur.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Contact yourdoctorimmediatelyif you experience any of the following serious side effects.
Prolonged or excessive bleeding.
Your doctor may decide to keep you under closer observation or change your treatment.
Side effects in a clinical study were reported with the following frequencies:
Very common:may affect more than 1 in 10 people
- gum bleeding
- fever
- tiredness
- headache
- nosebleeds
- rash
Common:may affect up to 1 in 10 people
eye bleeding
- vomiting blood
- blood in stools
- black and tarry stools
- stomach bleeding
- bleeding hemorrhoids
- rectal bleeding
- reactions at the injection site: rash, itching, and bleeding
- proven cerebral bleeding demonstrated by sudden severe headaches, vomiting, decreased level of consciousness, fever, sometimes convulsions, and neck stiffness or neck pain
- muscle pain
- stroke
- blood in urine
- excessive bleeding during periods
- vaginal bleeding
- coughing up blood
- shortness of breath
- bruising
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cablivi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after CAD/EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
Store in the original package to protect from light.
Cablivi can be stored at a temperature not above 25 °C for a single period of up to 2 months, but not beyond the expiry date. Cablivi should not be refrigerated again after being stored at room temperature. Never expose to temperatures above 30 °C.
Do not use Cablivi if you notice particles or color change before administration.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package contents and additional information
Cablivi contents
- vial powder
- The active substance is caplacizumab.
Each vial contains 10 mg of caplacizumab.
- The other ingredients are sucrose, anhydrous citric acid, disodium citrate dihydrate (see section 2 “Cablivi contains sodium”) and polysorbate 80.
- pre-filled syringe
The pre-filled syringe contains 1 ml of water for injections.
Appearance of Cablivi and package contents
Cablivi is provided as:
- a white powder for solution for injection in a glass vial, and
- water for injections in a pre-filled syringe to dissolve the powder.
After dissolving the powder in the solvent, the solution is clear, colorless or slightly yellowish.
Cablivi is available in
- individual packages containing 1 vial with caplacizumab powder, 1 pre-filled syringe with solvent, 1 vial adapter, 1 needle and 2 alcohol swabs
- multiple packages containing 7 individual packages
- multidose packages containing 7 vials with caplacizumab powder, 7 pre-filled syringes with solvent, 7 vial adapters, 7 needles and 14 alcohol swabs.
Only some pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Ablynx NV
Technologiepark 21
9052 Zwijnaarde
Belgium
You can request more information about this medicine from the local representative of the marketing authorisation holder.
België/Belgique/Belgien Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 | Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Luxembourg/Luxemburg Sanofi Belgium Tél/Tel: +32 (0)2 710 54 00 (Belgique/Belgien) |
Ceská republika Sanofi s.r.o. Tel: +420 233 086 111 | Magyarország SANOFI-AVENTIS Zrt. Tel.: +36 1 505 0050 |
Danmark Sanofi A/S Tlf: +45 45 16 70 00 | Malta Sanofi S.r.l. Tel: +39 02 39394275 |
Deutschland Sanofi-Aventis Deutschland GmbH Tel.: 0800 04 36 996 Tel. from abroad: +49 69 305 70 13 | Nederland Sanofi B.V. Tel: +31 20 245 4000 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Norge sanofi-aventis Norge AS Tlf: +47 67 10 71 00 |
Ελλάδα sanofi-aventis Μονοπρ?σωπη AEBE Τηλ: +30 210 900 16 00 | Österreich sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Polska Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
France Sanofi Winthrop IndustrieTél: 0 800 222 555 Appel depuis l’étranger : +33 1 57 63 23 23 | Portugal Sanofi - Produtos Farmacêuticos, Lda. Tel: +351 21 35 89 400 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | România Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tel: 800 536389 | Suomi/Finland Sanofi Oy Puh/Tel: +358 (0) 201 200 300 |
Κ?προς C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sverige Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50 | United Kingdom (Northern Ireland) sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44 (0) 800 035 2525 |
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The intravenous bolus injection of Cablivi administered at the start of treatment should be administered by a healthcare professional. The preparation of a dose of Cablivi for intravenous injection should be done in the same way as for a subcutaneous injection (see Instructions for use, steps 1 to 9, in section 3).
Cablivi can be administered intravenously by connecting the prepared syringe to standard Luer lock connections of intravenous lines or using an appropriate needle. The line can be flushed with a 9 mg/ml (0.9%) sodium chloride solution for injection.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CABLIVI 10 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1.5 mgActive substance: fondaparinuxManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: INJECTABLE, 2.5 mgActive substance: fondaparinuxManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: INJECTABLE, 5 mgActive substance: fondaparinuxManufacturer: Viatris Healthcare LimitedPrescription required
Online doctors for CABLIVI 10 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about CABLIVI 10 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















