
ARIXTRA 2.5 mg/0.5 mL Injectable Solution, Prefilled Syringe

How to use ARIXTRA 2.5 mg/0.5 mL Injectable Solution, Prefilled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Arixtra 2.5 mg/0.5 ml Solution for Injection
fondaparinux sodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Arixtra is and what it is used for
- What you need to know before you use Arixtra
- How to use Arixtra
- Possible side effects
- Storage of Arixtra
- Contents of the pack and further information
1. What Arixtra is and what it is used for
Arixtra is a medicine that helps prevent blood clots from forming in the blood vessels (an antithrombotic agent).
Arixtra contains a synthetic substance called fondaparinux sodium. This stops the effect of factor Xa "ten-A" in the blood and thus prevents the formation of unwanted blood clots (thrombi) in the blood vessels.
Arixtra is used to:
- prevent blood clots from forming in the blood vessels of the legs or lungs after orthopedic surgery, such as hip or knee surgery, or abdominal surgery
- prevent blood clots from forming during and shortly after a period of restricted mobility due to an acute illness.
- treat certain types of heart attack and severe angina (pain caused by narrowing of the heart arteries).
- treat blood clots in the blood vessels that are near the surface of the skin of the legs (superficial vein thrombosis)
2. What you need to know before you use Arixtra
Do not use Arixtra:
- if you are allergicto fondaparinux sodium or any of the other ingredients of this medicine (listed in section 6).
- if you are bleeding heavily
- if you have a bacterial infection of the heart
- if you have severe kidney disease.
- Tell your doctorif you think you may be affected by any of these conditions. If so, you must notuse Arixtra.
Arixtra.
Warnings and precautions
Talk to your doctor or pharmacist before you start using Arixtra:
- if you have had complications in the past during treatment with heparin or heparin-like medicines that cause a decrease in platelet count (heparin-induced thrombocytopenia)
- if you have a risk of uncontrolled bleeding(hemorrhage), such as:
- stomach ulcer
- bleeding disorders
- recent brain bleeding(intracranial hemorrhage)
- recent brain, spinal, or eye surgery
- if you have severe liver disease
- if you have kidney disease
- if you are 75 years or older
- if you weigh less than 50 kg
- Tell your doctorif you are affected by any of these conditions.
Children and adolescents
Arixtra has not been tested in children and adolescents under 17 years.
Using Arixtra with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription. The use of other medicines may affect how Arixtra works or be affected by Arixtra.
Pregnancy and breastfeeding
Arixtra should not be prescribed to pregnant women unless strictly necessary. Breastfeeding is not recommended during treatment with Arixtra. If you are pregnant, breastfeeding, think you may be pregnant, or are planning to become pregnant, consult your doctor or pharmacist before using this medicine.
Arixtra contains sodium
This medicine contains less than 23 mg of sodium per dose; it is essentially "sodium-free".
The Arixtra syringe may contain latex
The needle protector of the syringe may contain latex, which may cause allergic reactions in people sensitive to latex.
- Tell your doctorif you are allergic to latex before being treated with Arixtra.
3. How to use Arixtra
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is 2.5 mg once a day, injected at approximately the same time every day.
If you have kidney disease, the dose may be reduced to 1.5 mg once a day.How to administer Arixtra
- Arixtra is administered by injection under the skin (subcutaneously) in a skin fold formed in the lower abdomen area. The syringes are pre-filled with the exact dose you need. There are different syringes for the 2.5 mg and 1.5 mg doses.For a detailed description of how to use Arixtra, see the end of the leaflet. To treatsome types of heart attacks, a healthcare professional may administer the first dose in a vein (intravenously).
- Do notinject Arixtra into a muscle.
How long you should use Arixtra
You should use Arixtra for the period of time your doctor has told you, as Arixtra prevents you from having a serious illness.
If you inject more Arixtra than you should
Contact your doctor or pharmacist immediately because there is a higher risk of bleeding.If you forget to use Arixtra
- Administer the dose as soon as you remember. Do not inject a double dose to make up for forgotten doses.
- In case of doubt, contact your doctor or pharmacist.
If you stop using Arixtra
If you stop treatment before your doctor has told you to, you are at risk of developing a blood clot in a vein in your leg or lung. Before stopping treatment,contact your doctor or pharmacist.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Symptoms to look out for
Severe allergic reactions (anaphylaxis):are very rare (up to 1 in 10,000) in patients using Arixtra. The symptoms include:
- swelling, sometimes of the face or mouth (angioedema), which causes difficulty swallowing or breathing
- collapse.
- Contact a doctor immediatelyif you experience these symptoms. Stop using Arixtra.
Common side effects
These may affect more than 1 in 100 patientstreated with Arixtra.
- bleeding(e.g., from the area where the operation was performed, from a stomach ulcer, nosebleeds, bleeding gums, blood in the urine, coughing up blood, eye bleeding, bleeding into the joints, internal bleeding in the uterus)
- localized accumulation of blood(in any organ or body tissue)
- anemia(a reduction in the number of red blood cells)
- bruising
Uncommon side effects
These may affect up to 1 in 100 patientstreated with Arixtra.
- swelling (edema)
- feeling or being sick (nausea or vomiting)
- headache
- pain
- chest pain
- difficulty breathing
- skin rash or itching
- wound secretion
- fever
- reduction or increase in the number of platelets (blood cells needed for clotting)
- increase in some chemicals (enzymes) produced by the liver.
Rare side effects
These may affect up to 1 in 1,000 patientstreated with Arixtra.
- allergic reaction (including itching, swelling, rash)
- internal bleeding in the brain, liver, or abdomen
- anxiety or confusion
- fainting or dizziness, low blood pressure
- drowsiness or fatigue
- flushing
- cough
- leg pain or stomach pain
- diarrhea or constipation
- indigestion
- pain and inflammation at the injection site
- wound infection
- increase in bilirubin (a substance produced by the liver) in the blood
- increase in the amount of non-protein nitrogen in the blood
- reduction of potassium in the blood
- pain around the upper stomach or heartburn
If you experience side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Arixtra
- Keep this medicine out of the sight and reach of children
- Store below 25°C. Do not freeze
- It is not necessary to store Arixtra in the refrigerator.
Do not use this medicine:
- after the expiry date stated on the label and carton
- if you notice particles in the solution or if the solution is discolored
- if you notice that the syringe is damaged
- if you have opened the syringe and are not going to use it immediately.
Disposal of syringes:
Medicines or syringes should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines or syringes no longer required. This will help protect the environment.
6. Container Content and Additional Information
Arixtra Composition
- The active ingredient is 2.5 mg of fondaparinux sodium in 0.3 ml of injectable solution
- The other components are sodium chloride, water for injectable preparations, and hydrochloric acid and/or sodium hydroxide to adjust the pH (see section 2).
Arixtra does not contain any animal products.
Product Appearance and Container Content
Arixtra is a clear and colorless injectable solution. It is presented in a pre-filled syringe for single use, equipped with a safety system that helps prevent accidental needlesticks after use. It is available in packs of 2, 7, 10, and 20 pre-filled syringes. Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Viatris Healthcare Limited, Damastown Industrial Park, Mulhuddart, Dublin 15, DUBLIN, Ireland
Manufacturer:
Aspen Notre Dame de Bondeville, 1 rue de l'Abbaye, F-76960 Notre Dame de Bondeville, France.
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder.
Belgium/Belgique/Belgien Viatris Tel: + 32 (0)2 658 61 00 Bulgaria ?????? ???? ?: +359 2 44 55 400 Czech Republic Viatris CZ s.r.o. Tel: + 420 222 004 400 | Lithuania Viatris UAB Tel: +370 5 205 1288 Luxembourg/Luxemburg Viatris Tel: + 32 (0)2 658 61 00 (Belgium/Belgien) Hungary Viatris Healthcare Kft. Tel: + 36 1 465 2100 |
Denmark Viatris ApS Tel: +45 28 11 69 32 | Malta V.J. Salomone Pharma Ltd Tel: + 356 21 22 01 74 |
Germany Viatris Healthcare GmbH Tel: +49 800 0700 800 | Netherlands Mylan Healthcare BV Tel: +31 (0)20 426 3300 |
Estonia Viatris OÜ Tel: + 372 6363 052 | Norway Viatris AS Tel: + 47 66 75 33 00 |
Greece Viatris Hellas Ltd Tel: +30 2100 100 002 | Austria Mylan Österreich GmbH Tel: +43 1 86390 |
Spain Viatris Pharmaceuticals, S.L. Tel: +34 900 102 712 | Poland Viatris Healthcare Sp. z o.o. Tel: + 48 22 546 64 00 |
France Viatris Santé Tel: + 33 (0)4 37 25 75 00 | Portugal Viatris Healthcare, Lda. Tel: + 351 21 412 72 00 |
Croatia Viatris Hrvatska d.o.o. Tel: +385 1 23 50 599 | Romania BGP Products SRL Tel: +40 372 579 000 |
Ireland Mylan Ireland Limited Tel: +353 1 8711600 | Slovenia Viatris d.o.o. Tel: + 386 1 23 63 180 |
Iceland Icepharma hf Tel: +354 540 8000 | Slovakia Viatris Slovakia s.r.o. Tel: +421 2 32 199 100 |
Italy Viatris Italia S.r.l. Tel: + 39 02 612 46921 | Finland Viatris Oy Tel: +358 20 720 9555 |
Cyprus Varnavas Hadjipanayis Ltd Tel: +357 2220 7700 | Sweden Viatris AB Tel: + 46 (0)8 630 19 00 |
Latvia Viatris SIA Tel: +371 676 055 80 | United Kingdom (Northern Ireland) Mylan IRE Healthcare Limited +353 18711600 |
Belgium/Belgique/Belgien Mylan EPD bvba/sprl Tel: + 32 (0)2 658 61 00 | Luxembourg/Luxemburg Mylan EPD bvba/sprl Tel: + 32 (0)2 658 61 00 (Belgium/Belgien) |
Bulgaria ?????? ???? ?: +359 2 44 55 400 | Hungary Mylan EPD Kft Tel: + 36 1 465 2100 |
Czech Republic Mylan Healthcare CZ s.r.o. Tel: + 420 222 004 400 | Malta V.J. Salomone Pharma Ltd Tel: + 356 21 22 01 74 |
Denmark Viatris ApS Tel: +45 28 11 69 32 | Netherlands Mylan Healthcare BV Tel: +31 (0)20 426 3300 |
Germany Mylan Healthcare GmbH Tel: +49 800 0700 800 | Norway Mylan Healthcare Norge AS Tel: + 47 66 75 33 00 |
Estonia BGP Products Switzerland GmbH Eesti filiaal Tel: + 372 6363 052 | Austria Mylan Österreich GmbH Tel: +43 1 86390 |
Greece BGP ΠΡΟ?ΟΝΤΑ Μ.Ε.Π.Ε. Tel: +30 210 9891 777 | Poland Mylan Healthcare Sp. z o.o. Tel: + 48 22 546 64 00 |
Spain Mylan Pharmaceuticals, S.L. Tel: +34 900 102 712 | Portugal BGP Products, Unipessoal, Lda. Tel: + 351 21 412 72 56 |
France Viatris Santé Tel: + 33 (0)4 37 25 75 00 | Romania BGP Products SRL Tel: +40 372 579 000 |
Croatia Mylan Hrvatska d.o.o. Tel: +385 1 23 50 599 | Slovenia Mylan Healthcare d.o.o. Tel: + 386 1 23 63 180 |
Ireland Mylan Ireland Limited Tel: +353 1 8711600 | Slovakia Mylan s.r.o. Tel: +421 2 32 199 100 |
Iceland Icepharma hf Tel: +354 540 8000 | Finland Viatris Oy Tel: +358 20 720 9555 |
Italy Mylan Italia S.r.l. Tel: + 39 02 612 46921 | Sweden Mylan AB Tel: + 46 855 522 750 |
Cyprus Varnavas Hadjipanayis Ltd Tel: +357 2220 7700 | United Kingdom(Northern Ireland) Mylan IRE Healthcare Limited +353 18711600 |
Latvia Mylan Healthcare SIA Tel: +371 676 055 80 | |
Lithuania Mylan Healthcare UAB Tel: +370 5 205 1288 |
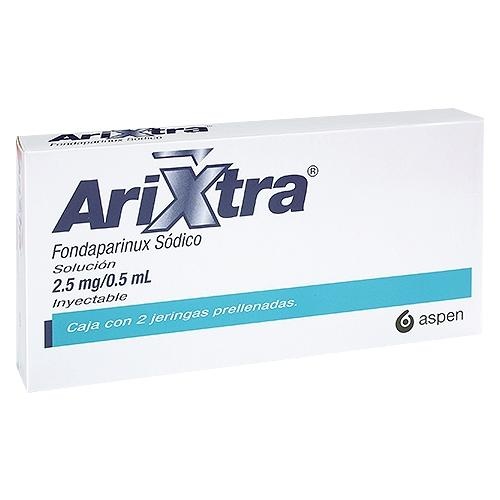
Drawing 3. Syringe with a manualneedle protection system with the safety cap covering the needle AFTER USE
USE
Types of Safety Syringes:
There are two types of safety syringes for Arixtra, designed to protect against accidental needlesticks after use. One type of syringe has an automaticneedle protection system and the other has a manualsystem.
Syringe Components:
- Needle protector
? Plunger
? Grip area (with fingers)
- Needle safety cap
Drawing 1. Syringe with an automaticneedle protection system

Syringe with a manualneedle protection system
Drawing 2. Syringe with a manualneedle protection system
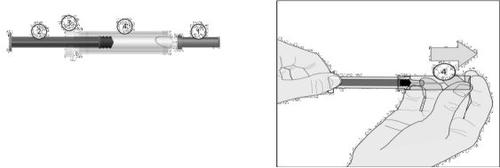
DESCRIPTION OF ARIXTRA USE MODEInstructions for Use
These instructions apply to both types of syringes (with automatic and manual needle protection systems).
When there is a different instruction between syringes, it will be clearly specified.
- Wash your handscarefully with water and soap and dry them with a towel.
- Remove the syringe from the packaging and check that:
- the expiration date has not passed
- the solution is transparent and colorless and does not contain particles
- the syringe has not been opened or damaged
- Sit or lie down in a comfortable position.Select a point in the lower abdomen (belly), at least 5 cm below the navel (drawing A).
For each injection, alternate the left and right sidesof the lower abdomen. This will help reduce discomfort at the injection site.
If injection in the lower abdomen is not possible, ask your doctor.
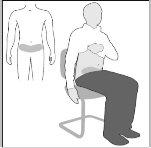
Drawing A
- Clean the injection area with a swab or cotton ball impregnated with alcohol.
- Remove the needle protector: first by twisting (drawing B1) and then by pulling it out from the syringe body (drawing B2).
Discard the needle protector.
Important Note
- Do not touch the needleand prevent it from coming into contact with any surface before injection.
- It is normal to find a small air bubble in the syringe. Do not try to remove this air bubblebefore administering the injectionas you may lose part of the medication.
- Gently pinch the skin you previously cleanedto form a fold. Hold the fold between your thumb and index finger throughout the injection (drawing C).
- Hold the syringe firmly by the grip area. Insert the needle into the skin fold at a right angle (drawing D).
Drawing B1
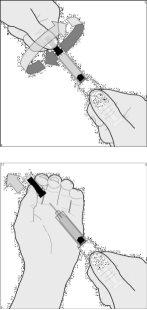
Drawing B2
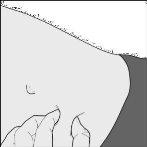
Drawing C
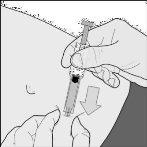
Drawing D
- Inject the entire contents of the syringeby pressing the plunger down to the maximum (drawing E).
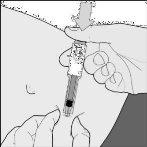
Drawing E
Syringe with automatic system
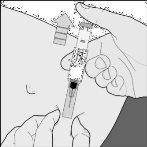
- Release the plungerand the needle will automatically move from the skin to a safety cap where it will be permanently locked (drawing F).
Drawing F
Syringe with manual system
- After injection, hold the syringe by the needle safety cap with the fingers of one hand, grasp the grip area with the fingers of the other hand, and pull back. This action releases the cap. Slide the cap along the syringe body until it is locked in a position that covers the needle, as shown in drawing 3.
Do not dispose of the used needle in the trash can. Dispose of it according to the instructions given by your doctor or pharmacist.
- Country of registration
- Average pharmacy price87.9 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ARIXTRA 2.5 mg/0.5 mL Injectable Solution, Prefilled SyringeDosage form: INJECTABLE, 1.5 mgActive substance: fondaparinuxManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: INJECTABLE, 5 mgActive substance: fondaparinuxManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: INJECTABLE, 7.5 mgActive substance: fondaparinuxManufacturer: Viatris Healthcare LimitedPrescription required
Online doctors for ARIXTRA 2.5 mg/0.5 mL Injectable Solution, Prefilled Syringe
Discuss questions about ARIXTRA 2.5 mg/0.5 mL Injectable Solution, Prefilled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















