
NIVESTIM 48 MU/0,5 ml SOLUÇÃO INJETÁVEL OU PARA PERFUSÃO


Como usar NIVESTIM 48 MU/0,5 ml SOLUÇÃO INJETÁVEL OU PARA PERFUSÃO
Introdução
Prospecto: informação para o utilizador
Nivestim 12MU/0,2ml solução injetável e para perfusão
Nivestim 30MU/0,5ml solução injetável e para perfusão
Nivestim 48MU/0,5ml solução injetável e para perfusão
filgrastim
Leia todo o prospecto atentamente antes de começar a usar o medicamento,pois contém informações importantes para si.
- Conserva este prospecto, pois pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, enfermeiro ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas a si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, pois pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Nivestim e para que é utilizado
- O que precisa saber antes de começar a usar Nivestim
- Como usar Nivestim
- Posíveis efeitos adversos
- Conservação de Nivestim
- Conteúdo do envase e informação adicional
1. O que é Nivestim e para que é utilizado
Nivestim é um fator de crescimento de glóbulos brancos (fator estimulante das colônias de granulócitos) e pertence a um grupo de medicamentos chamados citocinas. Os factores de crescimento são proteínas que se produzem de forma natural no corpo, mas também podem ser produzidos usando engenharia genética para uso como medicamento. Nivestim funciona fazendo com que a medula óssea produza mais glóbulos brancos.
Uma redução no número de glóbulos brancos (neutropenia) pode ocorrer por várias razões e torna o seu corpo menos eficaz na luta contra infecções. Nivestim estimula a medula óssea para produzir novos glóbulos brancos rapidamente.
Nivestim pode ser utilizado:
- para aumentar o número de glóbulos brancos após o tratamento com quimioterapia para ajudar a prevenir infecções;
- para aumentar o número de glóbulos brancos após um transplante de medula óssea para ajudar a prevenir infecções;
- antes da quimioterapia a doses altas para fazer com que a medula óssea produza mais células-mãe, que podem ser recolhidas e administradas de novo a si após o tratamento. Estas células podem ser recolhidas de si ou de um doador. As células-mãe voltarão então à medula óssea e produzirão células sanguíneas;
- para aumentar o número de glóbulos brancos se sofre de neutropenia crónica grave para ajudar a prevenir infecções;
- para ajudar a reduzir o risco de infecções em pacientes com infecção por VIH avançada.
2. O que precisa saber antes de começar a usar Nivestim
Não use Nivestim
- se é alérgico ao filgrastim ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Nivestim.
Informa ao seu médico antes de começar o tratamento se sofre:
- anemia de células falciformes, pois Nivestim pode causar crises de anemia falciforme;
- osteoporose (doença óssea).
Informa ao seu médico imediatamente durante o tratamento com Nivestim se:
- tiver sinais repentinos de alergia, tais como erupção, picazão ou urticária na pele, inchaço do rosto, lábios, língua ou outras partes do corpo, falta de ar, sibilância ou problemas ao respirar, pois podem ser sinais de uma reação alérgica grave (hipersensibilidade);
- experimenta inchaço no rosto ou tornozelos, sangue na urina ou urina de cor marrom, ou nota que urina com menor frequência do que o habitual (glomerulonefrite);
- experimenta dor na parte superior esquerda do abdômen (abdominal), dor abaixo da caixa torácica esquerda ou no extremo esquerdo do ombro (estes podem ser sintomas de um baço aumentado [esplenomegalia] ou uma possível ruptura do baço);
- nota sangramentos ou cardenales inusitados (estes podem ser sintomas de uma diminuição das plaquetas no sangue [trombocitopenia], com uma capacidade reduzida do sangue para coagular-se).
- Raramente foi notificada inflamação da aorta (o vaso sanguíneo grande que transporta sangue desde o coração até o resto do corpo) em pacientes com cancro e em doadores saudáveis. Os sintomas podem incluir febre, dor abdominal, mal-estar geral, dor de costas e marcadores inflamatórios aumentados. Informe ao seu médico se apresenta estes sintomas.
Perda de resposta ao filgrastim
Se experimenta uma perda de resposta ou se não se consegue manter a resposta ao tratamento com filgrastim, o seu médico investigará as causas, incluindo se desenvolveu anticorpos que possam neutralizar a atividade do filgrastim.
Pode ser que o seu médico queira supervisioná-lo estreitamente, ver secção 4 do prospecto.
Se é um paciente com neutropenia crónica grave, pode estar em risco de desenvolver cancro do sangue (leucemia, síndrome mielodisplásico [SMD]). Fale com o seu médico sobre os riscos de desenvolver cancro do sangue e das provas que devem ser realizadas. Se desenvolve ou é provável que desenvolva cancros do sangue, não deve utilizar Nivestim a menos que o indique o seu médico.
Se é um doador de células-mãe, deve ter entre 16 e 60 anos de idade.
Tenha especial cuidado com outros produtos que estimulam os glóbulos brancos
Nivestim pertence a um grupo de medicamentos que estimula a produção de glóbulos brancos. O seu médico deve registar sempre o produto exato que está a utilizar.
Uso de Nivestim com outros medicamentos
Informa ao seu médico ou farmacêutico se está a tomar, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
Gravidez e lactação
Nivestim não foi estudado em mulheres grávidas ou em período de amamentação.
Não se recomenda o uso de Nivestim durante a gravidez.
É importante que informe ao seu médico se:
- está grávida ou em período de amamentação;
- acredita que possa estar grávida; ou
- planeia ficar grávida.
Se fica grávida durante o tratamento com Nivestim, informe ao seu médico.
A menos que o seu médico lhe indique o contrário, deve deixar de amamentar se utiliza Nivestim.
Condução e uso de máquinas
A influência de Nivestim sobre a sua capacidade para conduzir e usar máquinas é pequena. Este medicamento pode produzir tonturas. É aconselhável esperar e ver como se sente após a administração de Nivestim antes de conduzir ou manejar maquinaria.
Nivestim contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por dose de 0,6 mg/ml ou 0,96 mg/ml; isto é, essencialmente “isento de sódio”.
Nivestim contém sorbitol
Este medicamento contém 50 mg de sorbitol em cada ml.
O sorbitol é uma fonte de frutose. Se si (ou o seu filho) sofre de intolerância hereditária à frutose (IHF), uma doença genética rara, não deve receber este medicamento. Os pacientes com IHF não podem descompor a frutose, o que pode provocar efeitos adversos graves.
Consulte com o seu médico antes de receber este medicamento se si (ou o seu filho) sofre de IHF ou se o seu filho não pode tomar alimentos ou bebidas doces porque lhe causam tonturas, vómitos ou efeitos desagradáveis como inchaço, cãibras no estômago ou diarreia.
3. Como usar Nivestim
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico, enfermeiro ou farmacêutico.
Como é administrado Nivestim e quanto devo tomar?
Nivestim é administrado por via geral uma vez ao dia como uma injeção no tecido justo abaixo da pele (conhecida como injeção subcutânea). Também pode ser administrado uma vez ao dia como uma injeção lenta na veia (conhecida como perfusão intravenosa). A dose habitual varia em função da sua doença e peso. O seu médico indicar-lhe-á a quantidade de Nivestim que deve tomar.
Pacientes com transplante de medula óssea após a quimioterapia:
Normalmente receberá a sua primeira dose de Nivestim pelo menos 24 horas após a quimioterapia e pelo menos 24 horas após receber o transplante de medula óssea.
A si ou às pessoas que o atendem pode ser ensinado a administrar injeções subcutâneas para que possa continuar o tratamento em casa. No entanto, não deve tentá-lo a menos que o seu profissional de saúde o tenha treinado de forma adequada.
Quanto tempo tenho que tomar Nivestim?
Terá que tomar Nivestim até que o seu recuento de glóbulos brancos seja normal. Ser-lhe-ão realizadas análises de sangue periódicas para supervisionar o número de glóbulos brancos no seu corpo. O seu médico indicar-lhe-á quanto tempo precisa tomar Nivestim.
Uso em crianças
Nivestim é utilizado para tratar crianças que estão a receber quimioterapia ou que sofrem de um recuento de glóbulos brancos baixo (neutropenia) grave. A dose em crianças que estão a receber quimioterapia é a mesma que para adultos.
Se usa mais Nivestim do que deve
Não aumente a dose que o seu médico lhe prescreveu. Se acredita que injetou uma dose maior do que a que devia, contacte o seu médico o mais rápido possível.
Se esqueceu de usar Nivestim
Se esqueceu de uma injeção, ou se se injetou menos dose, contacte o seu médico o mais rápido possível. Não tome uma dose dupla para compensar as doses esquecidas.
Se tiver alguma dúvida sobre o uso deste medicamento, pergunte ao seu médico, enfermeiro ou farmacêutico.
4. Posíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Informa ao seu médico imediatamentedurante o tratamento:
- se experimenta uma reação alérgica, incluindo fraqueza, queda da pressão arterial, dificuldade em respirar, inchaço do rosto (anafilaxia), erupção cutânea, erupção cutânea com picazão (urticária), inchaço do rosto, lábios, boca, língua ou garganta (angioedema) e falta de ar (dispnéia);
- se experimenta tosse, febre e dificuldade em respirar (dispnéia), pois pode ser um sinal do síndrome de distresse respiratório agudo (SDRA);
- se experimenta dano renal (glomerulonefrite). Foi observado dano renal em pacientes que recebiam filgrastim. Contacte com o seu médico imediatamente se observa inchaço no rosto ou tornozelos, sangue na urina ou urina de cor marrom, ou nota que urina com menor frequência do que o habitual;
- se experimenta algum dos seguintes efeitos adversos ou uma combinação deles:
- inchaço, que pode estar relacionado com urinar com uma menor frequência, dificuldade em respirar, inchaço e sensação de plenitude no abdômen, e uma sensação geral de cansaço. Estes sintomas geralmente se desenvolvem muito rapidamente.
Estes podem ser sintomas de uma doença chamada “síndrome de fuga capilar”, que provoca que o sangue se escape dos vasos sanguíneos pequenos para outros lugares do seu corpo e requer atenção médica urgente.
- Se experimenta uma combinação dos seguintes sintomas:
- febre, ou arrepios, ou sensação de muito frio, frequência cardíaca alta, confusão ou desorientação, dificuldade em respirar, dor extrema ou mal-estar e pele húmida ou suada.
Estes poderiam ser sintomas de uma afecção chamada “sepsis” (também chamada “intoxicação sanguínea”), uma infecção grave com resposta inflamatória de todo o corpo que pode ser potencialmente mortal e requer atenção médica urgente.
- se experimenta dor na parte superior esquerda do abdômen (abdominal), dor abaixo da caixa torácica esquerda ou dor no extremo esquerdo do ombro, pois se poderia tratar de algum problema com o seu baço (aumento do baço [esplenomegalia] ou rotura do baço);
- se está a ser tratado por neutropenia crónica grave e tem sangue na urina (hematúria). O seu médico realizar-lhe-á análises de urina periódicas se experimenta este efeito adverso ou se se encontram proteínas na sua urina (proteinúria).
Um efeito adverso frequente do uso de filgrastim é dor nos músculos ou ossos (dor musculoesquelética), que se pode evitar tomando medicamentos habituais para aliviar a dor (analgésicos). Nos pacientes submetidos a um transplante de células-mãe ou de medula óssea, pode aparecer doença do enxerto contra o hospedeiro (EICH). Esta é uma reação das células do doador contra o paciente que recebe o transplante; os sinais e sintomas incluem erupções nas palmas das mãos ou nas plantas dos pés, e úlceras e feridas na boca, intestino, fígado, pele, olhos, pulmões, vagina e articulações.
Em doadores saudáveis de células-mãe pode ser observado um aumento dos glóbulos brancos (leucocitose) e uma diminuição das plaquetas. Isto reduz a capacidade de coagulação do seu sangue (trombocitopenia). Estes efeitos serão vigilados pelo seu médico.
Efeitos adversos muito frequentes(podem afetar mais de 1 em cada 10 pessoas):
- Diminuição das plaquetas, o que reduz a capacidade do sangue de coagular-se (trombocitopenia)
- Recuento baixo de glóbulos vermelhos (anemia)
- Dor de cabeça
- Diarréia
- Vómitos
- Náuseas
- Perda ou enfraquecimento do cabelo inusual (alopecia)
- Cansaço (fadiga)
- Irritação e inchaço do revestimento do tubo digestivo, que vai da boca ao ânus (inflamação da mucosa)
- Febre (pirexia)
Efeitos adversos frequentes(podem afetar até 1 em cada 10 pessoas):
- Inflamação dos pulmões (bronquite)
- Infecção do tracto respiratório superior
- Infecção do tracto urinário
- Apetite diminuído
- Problemas para dormir (insónia)
- Tonturas
- Diminuição da sensibilidade, em especial na pele (hipoestesia)
- Formigueiro ou entorpecimento das mãos ou pés (parestesia)
- Tensão arterial baixa (hipotensão)
- Tensão arterial alta (hipertensão)
- Tosse
- Tosse com sangue (hemoptise)
- Dor na boca e garganta (dor orofaríngea)
- Hemorragia nasal (epistaxe)
- Prisão de ventre
- Dor oral
- Aumento do tamanho do fígado (hepatomegalia)
- Erupção
- Verdadeiro da pele (eritema)
- Cãibra muscular
- Dor ao urinar (disúria)
- Dor no peito
- Dor
- Debilidade geral (astenia)
- Sensação de mal-estar (mal-estar geral)
- Inchaço nas mãos e pés (edema periférico)
- Aumento de certas enzimas no sangue
- Mudanças na análise bioquímica do sangue
- Reação à transfusão
Efeitos adversos pouco frequentes(podem afetar até 1 em cada 100 pessoas):
- Aumento dos glóbulos brancos do sangue (leucocitose)
- Reação alérgica (hipersensibilidade)
- Rejeição do transplante de medula óssea (doença do enxerto contra o hospedeiro)
- Níveis altos de ácido úrico no sangue, que podem causar gota (hiperuricemia) (ácido úrico elevado no sangue)
- Dano hepático causado pelo bloqueio das pequenas veias do fígado (doença veno-oclusiva)
- Os pulmões não funcionam como devem, causando falta de ar (insuficiência respiratória)
- Inchaço ou fluido nos pulmões (edema pulmonar)
- Inflamação dos pulmões (doença pulmonar intersticial)
- Radiografias anormais dos pulmões (infiltração pulmonar)
- Sangramento dos pulmões (hemorragia pulmonar)
- Falta de absorção de oxigénio nos pulmões (hipóxia)
- Erupção cutânea irregular (erupção maculopapular)
- Doença que faz com que os ossos percam densidade, tornando-os mais frágeis e propensos a fracturas (osteoporose)
- Reação no local da injeção
Efeitos adversos raros(podem afetar até 1 em cada 1.000 pessoas):
- Dor intensa nos ossos, peito, intestinos ou articulações (anemia de células falciformes com crise)
- Reações alérgicas repentinas e que podem pôr em perigo a vida (reação anafiláctica)
- Inchaço e dor nas articulações, semelhante à gota (pseudogota)
- Uma mudança na forma como o seu corpo regula os fluidos corporais que pode resultar em inchaço (alterações do volume de fluidos)
- Inflamação dos vasos sanguíneos da pele (vasculite cutânea)
- Úlceras dolorosas, inflamadas e de cor vermelha escura nas extremidades e, por vezes, no rosto e no pescoço que cursam com febre (síndrome de Sweet)
- Piora da artrite reumatoide
- Mudanças anormais na urina
- Diminuição da densidade óssea
- Inflamação da aorta (o vaso sanguíneo grande que transporta sangue desde o coração até o resto do corpo), ver secção 2.
- Formação de células sanguíneas fora da medula óssea (hematopoese extramedular).
Comunicação de efeitos adversos
Se experimenta efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos possíveis adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Nivestim
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na caixa e na etiqueta da seringa pré-carregada após CAD. A data de validade é o último dia do mês que se indica.
Conservar e transportar refrigerado (entre 2°C e 8°C). Não congelar. Conservar a seringa pré-carregada no embalagem exterior para protegê-la da luz.
A seringa pode ser retirada da geladeira e deixada à temperatura ambiente durante um período único máximo de até 15 dias (mas a uma temperatura não superior a 25 °C).
Não empregue este medicamento se vir que o conteúdo da seringa está turvo ou se tem partículas.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos invólucros e dos medicamentos que não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Nivestim
- O princípio ativo é filgrastim. Cada ml contém 60 milhões de unidades [MU] (600 µg) ou 96 milhões de unidades ([MU] (960 µg) de filgrastim.
- Nivestim 12 MU/0,2 ml solução injetável/perfusão: cada seringa pré-carregada contém 12 milhões de unidades (MU), 120 µg de filgrastim em 0,2 ml (correspondentes a 0,6 mg/ml
- Nivestim 30 MU/0,5 ml solução injetável / perfusão: cada seringa pré-carregada contém 30 milhões de unidades (MU), 300 µg de filgrastim em 0,5 ml (correspondentes a 0,6 mg/ml).
- Nivestim 48 MU/0,5 ml solução injetável / perfusão: cada seringa pré-carregada contém 48 milhões de unidades (MU), 480 µg de filgrastim em 0,5 ml (correspondentes a 0,96 mg/ml
- Os demais componentes são ácido acético (glacial), hidróxido de sódio, sorbitol E420, polissorbato 80 e água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
Nivestim é uma solução para injeção/perfusão transparente e incolor que se fornece em uma seringa pré-carregada com uma agulha de injeção (aço inoxidável) com uma proteção para a agulha. O capuchão da agulha contém epoxipreno, um derivado do látex de borracha natural que pode entrar em contato com a agulha.
Nivestim encontra-se disponível em envases de 1, 5, 8 ou 10 seringas em cada envase. Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Titular da autorização de comercialização
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelas
Bélgica
Responsável pela fabricação
Hospira Zagreb d.o.o.
Prudnicka cesta 60
10291 Prigorje Brdovecko
Croácia
Podem solicitar mais informações respeito a este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica Luxemburgo Pfizer NV/SA Tel: +32 (0)2 554 62 11 | Lituânia Pfizer Luxembourg SARL filial em Lituânia Tel: +370 52 51 4000 |
| Hungria Pfizer Kft. Tel.: +36 1 488 37 00 |
República Checa Pfizer, spol. s r.o. Tel: +420-283-004-111 | Malta Drugsales Ltd Tel: +356 21 419 070/1/2 |
Dinamarca Pfizer ApS Tlf.: +45 44 20 11 00 | Países Baixos Pfizer bv Tel: +31 (0)800 63 34 636 |
Alemanha PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Noruega Pfizer AS Tlf: +47 67 52 61 00 |
Estônia Pfizer Luxembourg SARL filial na Estônia Tel: +372 666 7500 | Áustria Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Grécia Pfizer ΕΛΛΑΣ Α.Ε. Τηλ: +30 210 6785 800 | Polônia Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
Espanha Pfizer, S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 55 00 |
França Pfizer Tél: +33 (0)1 58 07 34 40 | Romênia Pfizer România S.R.L. Tel: +40 (0)21 207 28 00 |
Croácia Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Eslovênia Pfizer Luxembourg SARL Pfizer, filial para consultoria em farmacêutica, Liubliana Tel: +386 (0)1 52 11 400 |
Irlanda Pfizer Healthcare Ireland Unlimited Company Tel: +1800 633 363 (gratuito) Tel: +44 (0) 1304 616161 | República Eslovaca Pfizer Luxembourg SARL, filial Tel: +421–2–3355 5500 |
Islândia Icepharma hf. Sími: +354 540 8000 | Finlândia Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Itália Pfizer S.r.l. Tel: +39 06 33 18 21 | Suécia Pfizer AB Tel: +46 (0)8 550 520 00 |
Chipre Pfizer ΕΛΛΑΣ Α.Ε. (filial no Chipre) Τηλ: +357 22 817690 | Letônia Pfizer Luxembourg SARL filial na Letônia Tel: + 371 670 35 775 |
Data da última revisão deste prospecto: {MM/AAAA}.
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: https://www.ema.europa.eu.
-------------------------------------------------------------------------------------------------------
Instruções de autoadministração pelo paciente
Esta seção contém informações sobre como se administrar uma injeção de Nivestim. É importante que não tente se administrar a injeção sem que antes seu médico ou profissional de enfermagem tenha explicado como fazer. É também importante que descarte as seringas em um recipiente para objetos pontiagudos (à prova de picadas). Se não estiver seguro de querer se administrar a injeção ou tiver alguma dúvida, consulte seu médico ou profissional de enfermagem.
Como devo me injetar Nivestim?
Nivestim é administrado geralmente uma vez ao dia por meio de uma injeção, normalmente no tecido situado abaixo da pele. Isso é conhecido como injeção subcutânea.
Se aprender como se administrar a injeção, não precisará mais esperar em casa por uma enfermeira ou ir ao hospital ou clínica todos os dias para receber sua injeção.
Precisará se administrar a injeção aproximadamente no mesmo momento todos os dias. Os lugares mais adequados para a administração da injeção são os seguintes:
- A parte da frente dos músculos da coxa
- O abdômen, exceto a área ao redor do umbigo
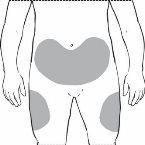
É melhor mudar todos os dias o local da injeção para evitar o risco de sensação dolorosa em qualquer lugar.
Equipamento necessário para a administração:
Para a administração da injeção subcutânea, são necessários os seguintes elementos:
- Uma seringa pré-carregada nova de Nivestim
- Um recipiente para objetos pontiagudos (à prova de picadas) para descartar as seringas usadas de forma segura
- Compressas antisépticas (se recomendadas por seu médico ou enfermeira)
Como administrar a injeção subcutânea de Nivestim?
- Certifique-se de administrar a injeção aproximadamente na mesma hora todos os dias.
- Retire o envase com a seringa pré-carregada de Nivestim da geladeira.
- Retire o blister com a seringa pré-carregada do envase. Quando o envase contiver blisters com mais de uma seringa pré-carregada, corte o blister com uma seringa pré-carregada ao longo da parte perfurada, devolva o resto dos blisters com seringas pré-carregadas ao envase e retorne o envase à geladeira.
- Abrir o blister com a seringa pré-carregada removendo a tampa do blister. Retire a seringa pré-carregada do blister segurando-a pelo corpo da seringa.
- Nãosegure o capuchão gris da agulha nem o êmbolo.
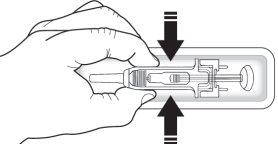
Verifique a seringa para se certificar de que o protetor de segurança da agulha cobre o corpo da seringa pré-carregada. Nãoempurre o protetor de segurança para a agulha sobre o capuchão da agulha antes da injeção. Isso pode ativar ou bloquear o protetor de segurança da agulha. Se o protetor de segurança da agulha cobre a agulha, significa que foi ativado.
Verifique que a solução é transparente, incolor e praticamente sem partículas visíveis. Nãoinspecione o produto através do plástico do dispositivo de segurança.
Verifique a data na etiqueta para se certificar de que o medicamento não passou da data de validade.
Certifique-se de ter um recipiente para objetos pontiagudos (à prova de picadas) por perto.
Deixe a seringa pré-carregada atingir a temperatura ambiente (aproximadamente 25 °C). Isso levará 15-30 minutos.
- Nãoretire o capuchão da agulha da seringa enquanto deixa a seringa pré-carregada atingir a temperatura ambiente.
- Nãoagite a seringa.
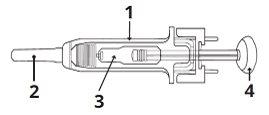
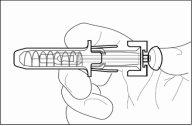
1 | Protetor de segurança para a agulha |
2 | Capuchão da agulha |
3 | Medicamento |
4 | Êmbolo |
- Não utilize a seringa de Nivestim se:
- O envase de cartão está aberto ou danificado.
- Falta o protetor de segurança para a agulha, foi removido ou foi ativado.
- O medicamento está turvo ou mudou de cor ou o líquido tem partículas flutuando nele.
- Alguma parte da seringa pré-carregada parece rachada ou quebrada ou líquido vazou da seringa.
- A seringa pré-carregada caiu. A seringa pré-carregada pode estar quebrada mesmo que não possa ver a quebra.
- Falta o capuchão da agulha ou não está bem colocado.
- A data de validade impressa na etiqueta passou.
Em todos os casos anteriores, descarte a seringa pré-carregada e use uma seringa pré-carregada nova.
- Encontre um local confortável para se administrar a injeção e verifique a dose que foi prescrita.
- Lave as mãos minuciosamente com água e sabão
- Pegue a seringa pré-carregada pelo corpo do protetor de segurança para a agulha com o capuchão da agulha apontando para cima.
- Nãosegure pela cabeça do êmbolo, o êmbolo ou o capuchão da agulha.
- Nãopuxe o êmbolo para trás em nenhum momento.
- Nãoretire o capuchão da agulha da seringa pré-carregada até que esteja pronto para injetar o medicamento.
- Retire o capuchão da agulha da seringa segurando o corpo da seringa e puxando o capuchão da agulha para fora e longe do corpo com cuidado, sem girá-lo. Descarte o capuchão da agulha. Não volte a colocar o capuchão da agulha. Não empurre o êmbolo, não toque a agulha nem agite a seringa.
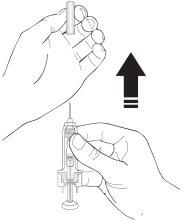
- A seringa agora está pronta para uso. Pode observar uma pequena bolha de ar na seringa. Não precisa expulsar a bolha de ar antes de injetar a solução. A injeção da solução com uma bolha de ar é inofensiva.
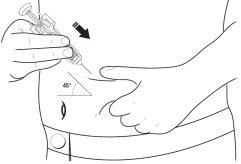
- Decida onde se vai administrar a injeção - Encontre um local na parte da frente do abdômen ou na parte da frente dos músculos da coxa. Escolha um local diferente de injeção cada vez. Não escolha uma área que esteja sensível, vermelha, danificada ou que tenha uma cicatriz. Limpe esta área da pele com uma gaze antiséptica.
- Pegue uma área ampla da pele, tendo cuidado para não tocar a área que havia limpo.
12 Com a outra mão, segure a seringa pré-carregada como se fosse um lápis. Use um movimento rápido de “dardo” para inserir a agulha em um ângulo aproximado de 45º na pele como mostrado.
- Puxe ligeiramente o êmbolo para confirmar que não perfurou um vaso sanguíneo. Se vir sangue na seringa, retire a agulha e insira em outro local. Mantendo a pele pega, pressione o êmbolo lentamente e uniformemente até que o conteúdo da seringa seja esvaziado.
- Depois de injetar a solução, retire a agulha da pele
- Certifique-se de que o protetor de seringa cubra a agulha de acordo com as instruções do protetor de seringa ativo ou do protetor de seringa passivo, conforme indicado abaixo.
- Não tente colocar o capuchão da agulha novamente. Coloque a seringa em um recipiente para objetos pontiagudos (à prova de picadas).
- Mantenha as seringas usadas fora do alcance e da vista das crianças
- Nuncacoloque as seringas usadas no contêiner de lixo doméstico normal.
Lembre-se
A maioria das pessoas pode aprender a se administrar a injeção subcutânea por si mesmas, mas se lhe resultar muito difícil, não hesite em pedir ajuda e conselho ao seu médico ou enfermeira.
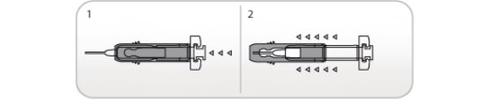
Uso do Protetor Ultrasseguro Ativo de agulhas para Nivestim 12 MU/0,2 ml solução injetável e para perfusão
As seringas pré-carregadas têm um Protetor Ultrasseguro Ativo de Agulhas para protegê-lo dos picos. Quando manusear uma seringa pré-carregada, mantenha as mãos atrás da agulha.
- Realize a injeção empregando a técnica descrita anteriormente
- Quando tiver completado a injeção, deslize a agulha dentro do protetor de segurança até que a agulha esteja completamente coberta (fará um “clic”).
Uso do Protetor Passivo Ultrasseguro de Agulha para Nivestim 30 MU/0,5 ml solução injetável / perfusão e Nivestim 48 MU/0,5 ml solução injetável / perfusão
As seringas pré-carregadas têm um Protetor Passivo Ultrasseguro de Agulha para protegê-lo dos picos. Quando manusear uma seringa pré-carregada, mantenha as mãos atrás da agulha.
- Realize a injeção empregando a técnica descrita anteriormente
- Pressione o êmbolo enquanto segura a seringa com os dedos até que a dose completa tenha sido administrada. O protetor de seringa passivo NÃO se ativará até que a dose TENHA SIDO ADMINISTRADA POR COMPLETO
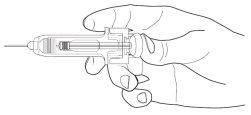
- Retire a agulha da pele, o êmbolo se moverá e permitirá que a seringa se mova para cima até que a agulha esteja guardada e bloqueada em seu lugar.
--------------------------------------------------------------------------------------------------------------------
Esta informação é destinada apenas a médicos ou profissionais do setor de saúde:
Nivestim não contém conservantes: tendo em vista o possível risco de contaminação microbiológica, as seringas de Nivestim são para uso único.
A exposição acidental a temperaturas de congelamento durante um máximo de 24 horas não afeta a estabilidade de Nivestim. As seringas pré-carregadas congeladas podem ser descongeladas e depois refrigeradas para uso futuro. Se a exposição tiver sido superior a 24 horas ou se as seringas tiverem sido congeladas mais de uma vez, então NÃO se deve usar Nivestim.
Nivestim não deve ser diluído com soluções de cloreto de sódio. Este medicamento não deve ser misturado com outros medicamentos que não sejam os que são mencionados a seguir. Filgrastim diluído pode ser adsorvido a materiais de plástico ou vidro, exceto se estiver diluído como mencionado a seguir.
Nivestim pode ser diluído, se necessário, em uma solução de glicose a 5%. Não se recomenda diluir a concentrações finais inferiores a 0,2 MU (2 µg) por mililitro. A solução deve ser inspecionada visualmente antes do uso. Apenas soluções transparentes sem partículas devem ser usadas. Nos pacientes tratados com filgrastim diluído a concentrações inferiores a 1,5 MU/ml (15 µg) por mililitro, deve ser adicionada albúmina sérica humana (ASH) até uma concentração final de 2 mg/ml.
Exemplo: se o volume de injeção final for de 20 ml e a dose total de filgrastim for inferior a 30 MU (300 µg), devem ser adicionados 0,2 ml de uma solução de albúmina sérica humana de 200 mg/ml (a 20%). Quando diluído em uma solução de glicose a 5%, Nivestim é compatível com o vidro e com vários plásticos, incluindo o cloreto de polivinilo, a poliolefina (um copolímero de polipropileno e polietileno) e o polipropileno.
Após a diluição: foi demonstrado que, durante o uso, a solução diluída para perfusão permanece fisicoquimicamente estável durante 24 horas a 2 - 8°C. Do ponto de vista microbiológico, o produto deve ser usado imediatamente. Se não for usado de forma imediata, os tempos de armazenamento durante o uso e as condições anteriores ao mesmo são responsabilidade do usuário e normalmente não devem ultrapassar as 24 horas a 2-8°C, a menos que a diluição tenha sido realizada em condições de assepsia validadas e controladas.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a NIVESTIM 48 MU/0,5 ml SOLUÇÃO INJETÁVEL OU PARA PERFUSÃOForma farmacêutica: INJETÁVEL, 12 UI/0,2 mlSubstância ativa: filgrastimFabricante: Accord Healthcare S.L.U.Requer receita médicaForma farmacêutica: INJETÁVEL, 0,3 mg / seringa pré-carregadaSubstância ativa: filgrastimFabricante: Accord Healthcare S.L.U.Requer receita médicaForma farmacêutica: INJETÁVEL, 0,3 mg / seringa pré-carregadaSubstância ativa: filgrastimFabricante: Accord Healthcare S.L.U.Requer receita médica
Alternativas a NIVESTIM 48 MU/0,5 ml SOLUÇÃO INJETÁVEL OU PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a NIVESTIM 48 MU/0,5 ml SOLUÇÃO INJETÁVEL OU PARA PERFUSÃO em Polónia
Alternativa a NIVESTIM 48 MU/0,5 ml SOLUÇÃO INJETÁVEL OU PARA PERFUSÃO em Ukraine
Médicos online para NIVESTIM 48 MU/0,5 ml SOLUÇÃO INJETÁVEL OU PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de NIVESTIM 48 MU/0,5 ml SOLUÇÃO INJETÁVEL OU PARA PERFUSÃO – sujeita a avaliação médica e regras locais.






