
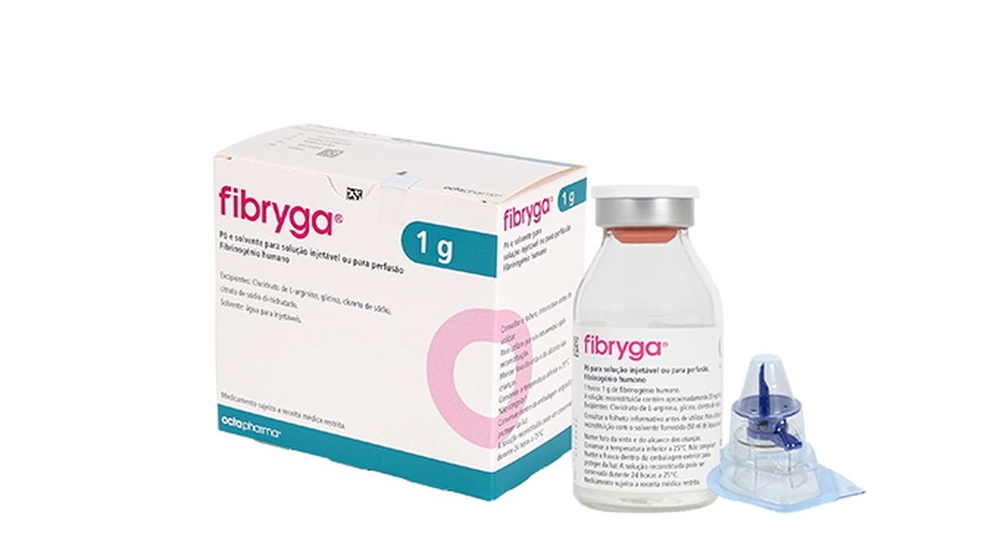
FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO

Pergunte a um médico sobre a prescrição de FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO

Como usar FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO
Introdução
Prospecto: informação para o utilizador
Fibryga 1g
Pó e dissolvente para solução injetável e para perfusão
Fibrinogénio humano

Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Fibryga e para que é utilizado
- O que precisa saber antes de começar a usar Fibryga
- Como usar Fibryga
- Efeitos adversos possíveis
- Conservação de Fibryga
- Conteúdo do envase e informações adicionais
1. O que é Fibryga e para que é utilizado
Fibryga contém fibrinogénio humano, que é uma proteína importante para a coagulação do sangue. A falta de fibrinogénio significa que o sangue não coagula tão bem como deveria, o que conduz a uma maior tendência a sangrar. A substituição do fibrinogénio humano por Fibryga corrigirá o defeito de coagulação.
Para que é utilizado Fibryga
Fibryga serve para:
- o tratamento de episódios hemorrágicos e profilaxia para a cirurgia em pacientes com uma falta de fibrinogénio congénita (hipo ou afibrinogenemia) com tendência a hemorragias.
- a suplementação de fibrinogénio em pacientes com hemorragias graves não controladas acompanhadas de uma falta de fibrinogénio adquirida durante a cirurgia.
.
2. O que precisa saber antes de começar a usar Fibryga
Não utilize Fibryga:
- se é alérgico ao fibrinogénio humano ou a algum dos outros componentes deste medicamento (incluídos na secção 6).
- se experimentou reações alérgicas a Fibryga no passado.
Por favor, informe o seu médico se é alérgico a algum medicamento.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a usar Fibryga.
Risco de coágulos nos vasos sanguíneos
O seu médico deve avaliar os benefícios deste medicamento face ao risco de coágulos nos vasos sanguíneos, em particular se:
- recebeu uma dose alta ou uma dose repetida deste medicamento
- teve um ataque cardíaco (antecedentes de doença coronária do coração ou infarto do miocárdio)
- padece uma doença hepática
- acaba de ser operado (pacientes pós-operatórios)
- se vai operar (pacientes perioperatórios)
- nos recém-nascidos (neonatos)
- é provável que sofra coágulos ou problemas de coagulação nos vasos sanguíneos (pacientes com risco de acontecimentos tromboembólicos ou coagulação intravascular disseminada).
O seu médico pode pedir-lhe que se faça análises de coagulação adicionais para controlar o risco.
Reações alérgicas e de tipo anafiláctico
Qualquer medicamento, como Fibryga, que se prepara a partir do sangue humano (que contém proteínas) e que se injeta numa veia (administrado por via intravenosa) pode causar reações alérgicas. Se experimentou reações alérgicas a Fibryga no passado, o seu médico aconselhá-lo-á se é necessário tomar antialérgicos.
O seu médico explicar-lhe-á os sinais de advertência das reações alérgicas ou de tipo anafiláctico.
Por favor, preste atenção aos primeiros sintomas das reações alérgicas (hipersensibilidade), como por exemplo:
- erupção cutânea
- eritema
- opressão no peito
- sibilância
- hipotensão,
- ou anafilaxia (quando algum ou todos os sintomas anteriores se desenvolvem rapidamente e são intensos).
Se se produzirem, a injeção ou perfusão de Fibryga deverá ser interrompida imediatamente (ou seja, interromper a injeção).
Segurança vírica
Quando os medicamentos se elaboram a partir do sangue ou plasma humanos, se põem em prática certas medidas para evitar que as infecções se transmitam aos pacientes. Estas incluem:
- seleção cuidadosa dos doadores de sangue e plasma para se asegurar de que se exclua os que apresentam risco de ser portadores de infecções
- a análise de cada doação e das misturas de plasma para detectar sinais de vírus ou infecções
- a inclusão de passos no processamento do sangue ou do plasma que podem inativar ou eliminar os vírus.
Apesar destas medidas, quando se administram medicamentos preparados a partir do sangue ou plasma humanos, não se pode excluir totalmente a possibilidade de transmitir uma infecção. Isto também é válido para qualquer vírus desconhecido ou emergente e outros tipos de infecções.
As medidas adoptadas se consideram eficazes para os vírus encapsulados como o vírus da imunodeficiência humana (VIH), o vírus da hepatite B e o vírus da hepatite C, e para o vírus não encapsulado da hepatite A. As medidas adoptadas podem ter um valor limitado contra os vírus não encapsulados como o parvovirus B19.
A infecção por parvovirus B19 pode ser grave nas mulheres grávidas (infecção do feto) e para os indivíduos cujo sistema imunológico está deprimido ou que têm alguns tipos de anemia (p. ex., anemia falciforme ou descomposição anormal dos glóbulos vermelhos).
Recomenda-se encarecidamente que cada vez que se receba uma dose de Fibryga, se registe o nome e o número de lote do produto para manter um registo dos lotes utilizados.
O seu médico pode recomendar-lhe que considere a possibilidade de se vacinar contra a hepatite A e B se receber regular ou repetidamente produtos com fibrinogénio derivado do plasma humano.
Crianças e adolescentes
Não há advertências ou precauções específicas ou adicionais aplicáveis às crianças e adolescentes.
Uso de Fibryga com outros medicamentos
Informa o seu médico ou farmacêutico se está utilizando, utilizou recentemente ou pudesse ter que utilizar qualquer outro medicamento.
Fibryga não se deve misturar com outros medicamentos, excepto os mencionados na secção “Esta informação está destinada unicamente a profissionais do sector sanitário / Reconstituição”.
Gravidez, lactação e fertilidade
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento. Este medicamento apenas deve ser utilizado durante a gravidez ou a lactação após consultar com o seu médico ou farmacêutico.
Condução e uso de máquinas
A influência de Fibryga sobre a capacidade para conduzir e utilizar máquinas é nula.
Fibryga contém sódio
Este medicamento contém 132 mg de sódio (componente principal da sal de mesa/para cozinhar) em cada frasco. Isto é equivalente a 6,6 % da ingestão diária máxima de sódio recomendada para um adulto. Tenha isto em conta se está seguindo uma dieta pobre em sódio.
3. Como usar Fibryga
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte de novo o seu médico.
Fibryga é administrado em forma de perfusão intravenosa (gotejamento numa veia) pelo pessoal sanitário.
A dose e a posologia dependem de:
- o seu peso
- a gravidade da sua doença
- a localização da hemorragia ou
- a natureza da sua operação e
- o seu estado de saúde
Uso em crianças e adolescentes
A administração de Fibryga em crianças e adolescentes (por via intravenosa) não difere da administração em adultos.
Se usar mais Fibryga do que deve
Para evitar o risco de sobredose, o seu médico realizará análises de sangue regulares para medir o seu nível de fibrinogénio.
Em caso de sobredose, o risco de coágulos anormais nos vasos sanguíneos pode aumentar.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
Forma de administração
Este medicamento deve ser injetado ou perfundido nas veias após a reconstituição com o dissolvente fornecido. Se tiver alguma outra dúvida sobre o uso deste produto, pergunte ao seu médico ou farmacêutico.
4. Efeitos adversos possíveis
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram.
Por favor, consulte o seu médico imediatamente:
- se se produzir algum dos efeitos adversos
- se observar algum efeito adverso que não aparece neste prospecto
Os seguintes efeitos adversos foram comunicados para Fibryga e outros medicamentos de fibrinogénio (a frequência dos efeitos adversos enumerados é desconhecida):
- Reações alérgicas e de tipo anafiláctico: reações cutâneas tais como erupção cutânea ou eritema da pele (ver a secção 2 “Advertências e precauções”)
- Cardiovasculares: inflamação das veias e formação de coágulos de sangue (ver a secção 2 “Advertências e precauções”)
- Aumento da temperatura corporal
Se experimentar algum dos sintomas anteriores, consulte o seu médico o mais breve possível.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es
Ao comunicar efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Fibryga
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na etiqueta e na caixa. A data de validade é o último dia do mês que se indica.
Não conserve a uma temperatura superior a 25 °C. Não congele. Mantenha o frasco no embalagem exterior para protegê-lo da luz.
O pó deve ser dissolvido apenas directamente antes da injeção ou perfusão. Demonstrou-se a estabilidade da solução reconstituída durante 24 horas a temperatura ambiente (máx. 25 °C). No entanto, para evitar a contaminação, a solução deve ser utilizada imediatamente e apenas uma vez. O produto reconstituído não se deve conservar na geladeira ou congelador.
Os medicamentos não se devem deitar pelos desagües nem para o lixo. Pergunte ao seu farmacêutico como se desfazer dos envases e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Fibryga
- O princípio ativo é fibrinogênio humano.
- Fibryga contém 1 g de fibrinogênio humano por frasco ou 20 mg/ml de fibrinogênio humano após a reconstituição com o dissolvente fornecido (50 ml de água para preparações injetáveis).
- Os outros componentes são cloreto de L-arginina, glicina, cloreto de sódio e citrato de sódio dihidratado.
Aspecto do produto e conteúdo do envase
Fibryga é apresentado como pó e dissolvente para solução injetável e para perfusão, e está disponível em frascos de vidro.
O pó é branco ou amarelo pálido e higroscópico, com aspecto também de massa friável.
O dissolvente é um líquido transparente e incolor.
A solução reconstituída é praticamente incolor e ligeiramente opalescente.
Fibryga é fornecido em uma caixa que contém:
- 1 frasco com o pó para solução injetável e para perfusão
- 1 frasco com o dissolvente (água para preparações injetáveis)
- 1 dispositivo de transferência nextaro
Título de autorização de comercialização
Octapharma, S.A.
Av. Castilla, 2 (P.E. San Fernando)
Ed. Dublín - 2ª Planta
28830 San Fernando de Henares
Madrid
Responsável pela fabricação
Octapharma Pharmazeutika Produktionsges.m.b.H.,
Oberlaaer Strasse 235, 1100 Vienna, Áustria
ou
Octapharma AB,
Lars Forssells gata 23, 112 75 Stockholm, Suécia
ou
Octapharma GmbH
Elisabeth-Selbert-Str. 11, 40764 Langenfeld, Alemanha
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu e no Reino Unido (Irlanda do Norte) com os seguintes nomes:
Fibryga®: Alemanha, Áustria, Bélgica, Bulgária, Chipre, Croácia, Dinamarca, Eslováquia, Espanha, Estónia, Finlândia, França, Hungria, Irlanda, Islândia, Itália, Letónia, Lituânia, Luxemburgo, Malta, Noruega, Países Baixos, Polónia, Portugal, Reino Unido (Irlanda do Norte), República Checa, Roménia, Suécia,
Fibrema®: Eslovénia
Data da última revisão deste prospecto: 11/2023
Outras fontes de informação
A informação detalhada deste medicamento está disponível no site da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) (http://www.aemps.gob.es/).
Esta informação está destinada unicamente a profissionais do setor sanitário:
Posologia
A dose e a duração do tratamento de substituição dependem da gravidade do distúrbio, bem como da localização e alcance das hemorragias e do estado clínico do paciente.
O nível de fibrinogênio (funcional) deve ser determinado para calcular a dose individual, e a quantidade e a frequência da administração devem ser determinadas em cada paciente mediante a medição regular do nível plasmático de fibrinogênio e a monitorização contínua do estado clínico do paciente e de outros tratamentos de substituição utilizados.
Em caso de intervenções de cirurgia maior, é essencial a monitorização precisa do tratamento de substituição mediante análise de coagulação.
- Profilação em pacientes com hipo ou afibrinogenemia congênita e tendência ao sangramento conhecida.
Para prevenir o sangramento excessivo durante os procedimentos quirúrgicos, é recomendado o tratamento profilático para elevar os níveis de fibrinogênio a 1 g/l e manter o fibrinogênio a este nível até que a hemostasia esteja assegurada e acima de 0,5 g/l até que a cicatrização da ferida seja completa.
Em caso de procedimento quirúrgico ou tratamento de um episódio hemorrágico, a dose deve ser calculada da seguinte maneira:
Dose (mg/kg de peso corporal) = [Nível objetivo (g/l) - nível medido (g/l)]
0,018 (g/l por mg/kg de peso corporal)
A posologia posterior (dose e frequência das injeções) deve ser adaptada em função do estado clínico do paciente e dos resultados de laboratório.
A meia-vida biológica do fibrinogênio é de 3-4 dias. Portanto, na ausência de consumo, não é necessário repetir o tratamento com fibrinogênio humano. Dada a acumulação que ocorre em caso de administração repetida para uso profilático, a dose e a frequência devem ser determinadas de acordo com os objetivos terapêuticos do médico para cada paciente determinado.
População pediátrica
Em caso de procedimento quirúrgico ou tratamento de um episódio hemorrágico, a dose em adolescentes deve ser calculada conforme a fórmula descrita anteriormente para os adultos, enquanto a dose em crianças <12 anos de idade deve ser calculada da seguinte maneira:
Dose (mg/kg de peso corporal) = [Nível objetivo (g/l) – nível medido (g/l)]
0,014 (g/l por mg/kg de peso corporal)
A posologia posterior deve ser adaptada em função do estado clínico do paciente e dos resultados de laboratório.
Pacientes de idade avançada
Os estudos clínicos com Fibryga não incluíram pacientes de 65 anos ou mais como para fornecer provas conclusivas sobre se esses pacientes respondem ou não de maneira diferente aos pacientes mais jovens.
- Tratamento de hemorragias
Hemorragia em pacientes com hipo ou afibrinogenemia congênita
Os episódios hemorrágicos devem ser tratados conforme as fórmulas anteriormente indicadas para adultos/adolescentes e crianças, respectivamente até alcançar um nível de fibrinogênio em plasma objetivo recomendado de 1 g/l. Este nível deve ser mantido até que a hemostasia esteja assegurada.
Hemorragia em pacientes com deficiência de fibrinogênio adquirida
Adultos
Geralmente são administrados 1-2 g no início, com perfusões posteriores conforme necessário. Em caso de hemorragia grave, por exemplo durante uma cirurgia maior, podem ser necessárias quantidades maiores (4-8 g) de fibrinogênio.
População pediátrica
A dose deve ser determinada de acordo com o peso corporal e as necessidades clínicas, embora normalmente seja de 20-30 mg/kg.
Instruções para a preparação e administração
Instruções gerais
- A solução reconstituída deve ser praticamente incolor e ligeiramente opalescente. Não use soluções turvas ou com sedimentos.
- Fibryga é válido unicamente para um único uso. Não reutilize nenhum dos componentes.
- Por motivos de segurança microbiológica, a solução deve ser administrada imediatamente após a reconstituição. Foi demonstrada a estabilidade química e física em condições de uso da solução reconstituída durante 24 horas a temperatura ambiente (máx. 25 °C). Após a reconstituição, não refrigere nem congele a solução de Fibryga.
Reconstituição
- Certifique-se de que o frasco de pó (Fibryga) e o frasco de dissolvente estão à temperatura ambiente. Esta temperatura deve ser mantida durante a reconstituição. Se um banho-maria for usado para aquecer, deve-se ter cuidado para evitar que a água entre em contato com os tampões de borracha ou com as cápsulas de fechamento flip-off dos envases. A temperatura da água não deve ultrapassar +37 °C.
- Retire as cápsulas de fechamento flip-off do frasco de pó (Fibryga) e do frasco de dissolvente para deixar à vista a parte central do tampão de perfusão. Limpe os tampões de borracha com uma toalha com álcool e deixe que sequem.
- Retire a tampa do envase do dispositivo de transferência (nextaro) (Fig. 1). Para manter a esterilidade, não extraia o dispositivo de transferência do blister transparente. Não toque no punção.

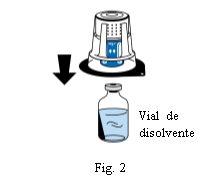
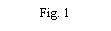 Coloque o frasco de dissolvente sobre uma superfície plana e limpa e segure-o firmemente. Sem retirar o blister, coloque a parte azul do dispositivo de transferência sobre o frasco de dissolvente. Pressione firmemente em linha reta para baixo até que encaixe em posição (Fig. 2). Não o gire ao acoplar.
Coloque o frasco de dissolvente sobre uma superfície plana e limpa e segure-o firmemente. Sem retirar o blister, coloque a parte azul do dispositivo de transferência sobre o frasco de dissolvente. Pressione firmemente em linha reta para baixo até que encaixe em posição (Fig. 2). Não o gire ao acoplar.
Nota:O dispositivo de transferência deve ser acoplado primeiro ao frasco de dissolvente e depois ao frasco de pó liofilizado. De outra forma, o vácuo será perdido e não ocorrerá a transferência do dissolvente.
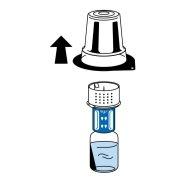
- Enquanto segura o frasco de dissolvente, retire com cuidado o blister do dispositivo de transferência (nextaro) puxando-o para cima na vertical. Certifique-se de deixar o dispositivo de transferência acoplado firmemente ao frasco de dissolvente (Fig. 3).

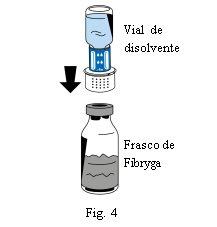
- Coloque o frasco de pó (Fibryga) sobre uma superfície plana e limpa e segure-o firmemente. Pegue o frasco de dissolvente com o dispositivo de transferência acoplado e vire-o. Coloque a parte branca do conector do dispositivo de transferência sobre o frasco de pó (Fibryga) e pressione firmemente para baixo até que encaixe em posição (Fig. 4). Não o gire ao acoplar. O dissolvente fluirá automaticamente para o frasco de pó (Fibryga).
- Com o frasco de dissolvente ainda acoplado, gire suavemente o frasco de Fibryga até que o pó se dissolva completamente. Para evitar que se forme espuma, não agite o frasco. O pó deve dissolver-se completamente em cerca de 5 minutos. O pó não deve levar mais de 20 minutos para dissolver. Se não se dissolver em 20 minutos, o produto deve ser descartado.
- Nas raras ocasiões em que se observa pó não reconstituído flutuando durante a transferência de água para preparações injetáveis ou nas quais o tempo de reconstituição se prolonga inesperadamente, pode-se favorecer o processo de dissolução agitando horizontalmente o frasco de forma mais enérgica.
- Uma vez completada a reconstituição, desrosqueie o dispositivo de transferência (parte azul) no sentido contrário ao das agulhas do relógio em duas partes (Fig. 5). Não toque no conector Luer da parte branca do dispositivo de transferência.
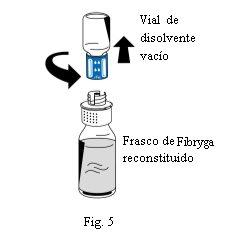
- Descarte o frasco de dissolvente vazio junto com a parte azul do dispositivo de transferência.
Administração
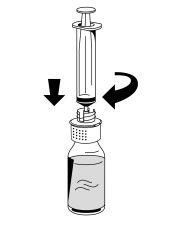
- Acople com cuidado uma seringa ao conector Luer da parte branca do dispositivo de transferência (Fig. 6).
- Vire o frasco de Fibryga e retire a solução para dentro da seringa (Fig. 7).
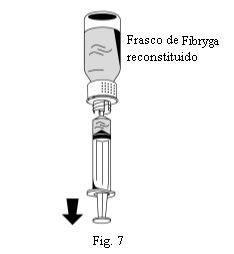

- Uma vez transferida a solução, segure firmemente o corpo da seringa (mantendo o êmbolo da seringa orientado para baixo) e retire a seringa do dispositivo de transferência (Fig. 8).
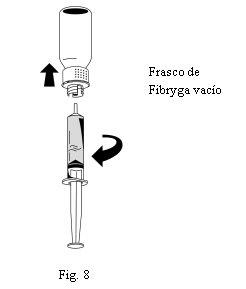
- Descarte a parte branca do dispositivo de transferência junto com o frasco de Fibryga vazio.
Recomenda-se um equipamento de perfusão padrão para a administração intravenosa da solução reconstituída à temperatura ambiente.
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a regulamentação local.
Forma de administração
Perfusão ou injeção intravenosa.
Fibryga deve ser administrado lentamente por via intravenosa a uma velocidade máxima recomendada de 5 ml por minuto em pacientes com hipo ou afibrinogenemia congênita, e a uma velocidade máxima recomendada de 10 ml por minuto em pacientes com deficiência de fibrinogênio adquirida.
Incompatibilidades
Este medicamento não deve ser misturado com outros.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃOForma farmacêutica: INJETÁVEL, 1,5 gSubstância ativa: fibrinogen, humanRequer receita médicaForma farmacêutica: INJETÁVEL, 1 g (20 mg/mL)Substância ativa: fibrinogen, humanFabricante: Csl Behring GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 1.000 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médica
Alternativas a FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO em Polónia
Alternativa a FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO em Ukraine
Médicos online para FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de FIBRYGA 1 g PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO – sujeita a avaliação médica e regras locais.














