
FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Pergunte a um médico sobre a prescrição de FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL

Como usar FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Introdução
Prospecto: informação para o utilizador
Fator IX Grifols 50 UI/mlpó e dissolvente para solução injetável
Fator IX de coagulação humano
Leia todo o prospecto atentamente antes de começar a usar o medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi-lhe prescrito somente a si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo doprospecto
- O que é Fator IX Grifols e para que é utilizado
- O que precisa saber antes de começar a usar Fator IX Grifols
- Como usar Fator IX Grifols
- Posíveis efeitos adversos
- Conservação de Fator IX Grifols
- Conteúdo do envase e informação adicional
1. O que é Fator IX Grifols e para que é utilizado
Fator IX Grifols é um medicamento que contém o fator IX de coagulação humana.
Fator IX Grifols pertence ao grupo de medicamentos denominado antihemorrágicos: fatores de coagulação sanguínea.
Fator IX Grifols está indicado para o tratamento e profilaxia (prevenção) de hemorragias em pacientes com hemofilia B (défice congénito de fator IX). Estes pacientes não têm suficiente fator IX funcional. Fator IX Grifols serve para aumentar a quantidade de fator IX no sangue, permitindo assim que o sangue coagule.
2. O que precisa saber antes de começar a usar Fator IX Grifols
Não useFator IX Grifols
Se é alérgico ao princípio ativo ou a algum dos outros componentes deste medicamento (incluídos na secção 6). Ver informação importante sobre alguns dos componentes de Fator IX Grifols no final desta secção.
Se tiver alguma dúvida sobre o anterior, consulte o seu médico.
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Fator IX Grifols.
- Existe a remota possibilidade de que possa experimentar uma reação anafiláctica (reação alérgica repentina grave). Se sentir opressão torácica, sensação de mareio, vertigem ou náusea, ou bem se mareia estando de pé, é possível que esteja a sofrer uma reação anafiláctica a Fator IX Grifols. Se isso ocorrer, interrompa imediatamente a administração do produto e peça assistência médica.
- Se aparecerem reações de hipersensibilidade (alergia p. ex. febre, urticária generalizada, opressão do peito, jadeios, hipotensão e anafilaxia), durante a administração de Fator IX Grifols, a injeção/perfusão deve ser interrompida. O seu médico decidirá sobre o tratamento adequado (p. ex. antihistamínicos, terapia de choque).
- É possível que o seu médico deseje realizar algum exame para garantir que a dose que recebe de Fator IX Grifols é suficiente para atingir e manter níveis adequados de fator IX.
- Se a sua hemorragia não for controlada com Fator IX Grifols, consulte o seu médico imediatamente. É possível que tenha desenvolvido inibidores do fator IX, pelo que o seu médico querrá realizar exames para confirmá-lo. Os inibidores do fator IX são anticorpos presentes no sangue que bloqueiam o fator IX que está a utilizar. Isso torna o fator IX menos eficaz no controlo do sangramento.
- Se tiver alguma doença com risco de trombose (histórico de doenças do coração ou infarto de miocárdio agudo, doença hepática, alterações tromboembólicas, distúrbios na coagulação, ou em crianças recém-nascidas) e se lhe for administrada uma dose alta de fator IX em casos de cirurgia maior. Com uma vigilância adequada, é possível detectar a tempo possíveis complicações e adotar as medidas oportunas. Algumas dessas complicações são p. ex. tromboembolismo e coagulopatia de consumo.
- Se precisar de um dispositivo de acesso venoso central (DAVC) para a administração de Fator IX Grifols, o seu médico deve considerar o risco de complicações relacionadas com o DAVC, incluindo infecções locais, presença de bactérias no sangue (bacteriemia) e a formação de um coágulo no vaso sanguíneo (trombose) onde se insere o catéter.
Quando os medicamentos são elaborados a partir de sangue ou plasma humano, devem ser adotadas um número de medidas para prevenir uma possível transmissão de infecções aos pacientes. Estas medidas incluem:
- uma seleção cuidadosa dos doadores de sangue e plasma para garantir a exclusão de doadores com risco de padecer infecções,
- a análise de cada doação e das misturas de plasma para detectar possíveis vírus ou infecções,
- a inclusão de uma série de etapas no processamento do sangue ou do plasma que podem inativar ou eliminar os vírus.
Apesar dessas medidas, quando se administram medicamentos preparados a partir de sangue ou plasma humano, não se pode excluir totalmente a possibilidade de transmissão de infecções. Isso é aplicável também a vírus desconhecidos ou emergentes e a outros tipos de infecções.
As medidas adotadas são consideradas eficazes para os vírus envoltos, como o vírus da imunodeficiência humana (VIH), o vírus da hepatite B e o vírus da hepatite C, e para o vírus não envolto da hepatite A. As medidas tomadas podem ter um valor limitado para vírus não envoltos, como o parvovirus B19.
A infecção por parvovirus B19 pode ser grave para uma mulher grávida (infecção fetal) e para pessoas cujo sistema imunológico está deprimido ou que padecem algum tipo de anemia (p. ex. com anemia falciforme ou com anemia hemolítica).
O seu médico pode recomendar que considere a vacinação contra hepatite A e B se receber regularmente/repetidamente concentrados de fator IX derivados de plasma humano.
Sempre que lhe for administrada uma dose de Fator IX Grifols, é recomendável deixar constância do nome e do número de lote do medicamento para manter um registo dos lotes utilizados.
Existe uma possível conexão entre a aparência de inibidores do fator IX e reações alérgicas. Os pacientes com inibidores do FIX podem ter um maior risco de reações anafilácticas. Por conseguinte, nos pacientes que sofram uma reação alérgica, deve ser investigada a presença de um inibidor do fator IX.
Uso de Fator IX Grifols com outros medicamentos
- Informa o seu médico ou farmacêutico se está a utilizar ou utilizou recentemente ou possa ter que utilizar qualquer outro medicamento.
- Atualmente não se conhecem interações com outros medicamentos.
- Fator IX Grifols não deve ser misturado com outros medicamentos antes da administração, porque pode afetar de forma adversa a eficácia e segurança do produto.
Gravidez, lactação e fertilidade
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
- Informa o seu médico se está grávida ou em período de lactação.
- O seu médico decidirá se Fator IX Grifols pode ser utilizado durante a gravidez e a lactação.
- Como a hemofilia B é rara na mulher, não se dispõe de experiência sobre o uso de Fator IX Grifols durante a gravidez e a lactação.
Condução e uso de máquinas
Não existe nenhum indício de que Fator IX Grifols possa afetar a capacidade de conduzir ou utilizar máquinas.
Conteúdo em sódio
Fator IX Grifols contém 20,7 mg de sódio na apresentação de Fator IX Grifols 250 UI/5 ml, 41,4 mg de sódio na apresentação de Fator IX Grifols 500 UI/10 ml, 82,8 mg de sódio na apresentação de Fator IX Grifols 1000 UI/20 ml e 124,2 mg de sódio na apresentação de Fator IX Grifols 1500 UI/30 ml. Isso é equivalente ao 1,04%, 2,07%, 4,14% e 6,21%, respectivamente, da máxima quantidade diária de sódio recomendada pela OMS para um adulto (2 g de sódio).
3. Como usar Fator IX Grifols
Reconstituir o produto como se descreve neste apartado. O produto deve ser administrado lentamente, especialmente a primeira dose (aproximadamente 3 ml/min) por via intravenosa.
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
O seu médico decidirá a dose de Fator IX Grifols que receberá. Esta dose e a sua duração dependerão das suas necessidades individuais de tratamento substitutivo com fator IX e da farmacocinética (recuperação e vida média), que deve ser comprovada regularmente.
O seu médico pode modificar a dose de Fator IX Grifols que recebe ao longo do tempo.
Dose para o tratamento
A dose necessária é determinada utilizando a fórmula seguinte:
Unidades necessárias = peso corporal (kg) x aumento desejado de fator IX (%) (UI/dl) x 0,8
Dose para profilaxia em hemorragias
Na profilaxia rotineira para impedir hemorragias em pacientes com hemofilia B grave, devem ser administradas doses de 20 a 40 UI de fator IX/kg de peso corporal a intervalos de 3 a 4 dias. Em alguns casos, especialmente em pacientes jovens, pode ser necessário reduzir os intervalos de administração ou doses mais elevadas.
Pacientes com inibidores
Se desenvolveu inibidores do FIX, pode precisar de uma maior quantidade de Fator IX Grifols para controlar a hemorragia. Se esta dose não controlar a hemorragia, o seu médico pode considerar o uso de outro medicamento alternativo. Não aumente a dose total de Fator IX Grifols que utiliza para controlar a sua hemorragia sem consultar o seu médico.
Instruções de uso/manuseio
Siga estas instruções, a menos que o seu médico lhe tenha dado outras indicações distintas.
Para a reconstituição e administração de Fator IX Grifols, apresentação de 1500 UI/30 ml, cujo dissolvente se apresenta em frascos, a preparação da solução é a seguinte:
- Aquecer os frascos sem ultrapassar os 37 ºC.
- Descartar o lacre do frasco de dissolvente, desinfetando o tampão com uma toalhita com álcool.
- Retirar do envase a agulha de dupla ponta. Separar um dos capuchões que protegem as pontas e perfurar o tampão do frasco de dissolvente.
- Descartar o lacre do frasco de produto liofilizado, desinfetando o tampão com uma toalhita com álcool.
- Separar o capuchão da outra ponta da agulha.
- Inverter o frasco de dissolvente e perfurar o frasco de liofilizado, assegurando-se de que seja trasvasado todo o dissolvente e evitando a perda de vácuo.
- Separar o frasco de dissolvente com a agulha de dupla ponta. Girar suavemente o frasco, procurando não produzir espuma, até a total dissolução. Não agitar.
- Retirar o filtro do blister e inseri-lo na seringa, recarregar a seringa com ar suficiente para o volume total da solução. Inserir a agulha no filtro e perfurar o frasco do produto reconstituído. Injetar o ar pré-carregado da seringa, através do filtro, para seguidamente inverter a posição do frasco e aspirar o conteúdo na seringa.
- Retirar o conjunto filtro-agulha e administrar lentamente por via intravenosa, utilizando a agulha borboleta fornecida, a uma velocidade de 3 ml/min.
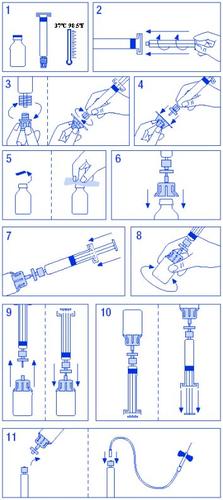
Para a reconstituição e administração de Fator IX Grifols, apresentações de 250 UI/5 ml, 500 UI/10 ml e 1000 UI/20 ml, em que o dissolvente se apresenta em seringas pré-carregadas, a preparação da solução é a seguinte:
- Aquecer o frasco e a seringa de dissolvente sem ultrapassar os 37 ºC.
- Acoplar o êmbolo à seringa de dissolvente.
- Descartar o filtro. Separar o tampão do cone da seringa de dissolvente e acoplá-la ao filtro.
- Descartar o adaptador de frasco e acoplá-lo ao conjunto filtro-seringa.
- Descartar o lacre do frasco, desinfetando o tampão com uma toalhita com álcool.
- Introduzir a espinha do adaptador no frasco.
- Trasvasar todo o dissolvente da seringa para o frasco.
- Girar suavemente o frasco, procurando não produzir espuma, até a total dissolução. Não agitar.
- Separar o conjunto filtro-seringa do resto. Aspirar ar suficiente para o volume total da solução. Acoplar novamente o conjunto filtro-seringa ao frasco.
- Inverter o frasco e aspirar o conteúdo na seringa.
- Separar a seringa e administrar lentamente por via intravenosa, utilizando a agulha borboleta fornecida, a uma velocidade de 3 ml/min.
É importante utilizar o equipamento para injeção fornecido com o medicamento. Em caso de utilização de equipamentos médicos de perfusão, verificar a compatibilidade do sistema com a seringa pré-carregada. Para assegurar uma administração do produto adequada, pode ser necessário, por vezes, utilizar um adaptador.
Esquema de reconstituição para o dissolvente em seringas
Se usar maisFator IX Grifolsdo que deve
Não foram comunicados casos de sobredosagem com fator IX de coagulação humano. No entanto, se utilizou Fator IX Grifols mais do que devia, consulte imediatamente o seu médico ou farmacêutico.
Em caso de sobredosagem ou administração acidental, consulte o Serviço de Informação Toxicológica, telefone 91 562 04 20.
Se esqueceu de usar Fator IX Grifols
- Não tome uma dose dupla para compensar as doses esquecidas.
- Continue com a próxima administração imediatamente e siga a intervalos regulares, tal como o seu médico indicou.
4. Posíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Em raros casos, pode notar algum destes efeitos adversos após a administração de Fator IX Grifols:
- Prurido, reações locais no local da injeção (p. ex. sensação de queimadura e rubor transitório)
- Reações alérgicas (p. ex. opressão torácica/sensação geral de mal-estar, mareio, náusea e ligeira queda de tensão que pode fazer com que se mareie estando de pé)
Também não pode excluir-se completamente a possibilidade de um choque anafiláctico. Se notar algum dos sintomas seguintes durante a injeção/perfusão
- opressão torácica/sensação geral de mal-estar
- mareio
- hipotensão ligeira (queda ligeira da pressão arterial com sensação de mareio estando de pé)
- náusea
pode ser um sinal precoce de hipersensibilidade e reação anafiláctica. Se ocorrer uma reação alérgica ou anafiláctica, a injeção/perfusão deve ser interrompida e consultar o seu médico imediatamente.
No entanto, não pode excluir-se totalmente a possibilidade de reações alérgicas aos componentes do preparado. A formação de anticorpos neutralizantes do fator IX (inibidores) é uma complicação conhecida no tratamento de pacientes com hemofilia B. O desenvolvimento de inibidores deve ser cuidadosamente monitorizado mediante exames de laboratório e exames clínicos apropriados para determinar a formação desses inibidores.
Existe o risco de complicações tromboembólicas com Fator IX Grifols, particularmente se tiver risco de trombose e/ou receber terapia a doses altas.
- Para informação sobre a segurança viral, ver secção 2.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Fator IX Grifols
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data que aparece no envase após a abreviatura “CAD”. A data de caducidade é o último dia do mês que se indica.
Frascos de pó liofilizado (fator IX de coagulação humano): conservar entre 2 ºC e 8 ºC (na geladeira).
Frascos ou seringas de dissolvente (água para injeções): conservar entre 2 ºC e 30 ºC.
Quando proceder à sua administração ambulatorial, o produto pode ser mantido à temperatura ambiente (não conservar acima de 25 ºC) durante um único período de 3 meses, no máximo.
O produto não deve voltar a ser refrigerado após estar conservado à temperatura ambiente.
Não utilize este medicamento se observar que a solução apresenta turbidez ou sedimentos. Geralmente, a solução é clara ou ligeiramente opalescente.
Uma vez reconstituída, a solução deve ser descartada se se observarem partículas no seu interior ou algum tipo de decoloração.
A solução reconstituída deve ser utilizada imediatamente ou no prazo de 3 horas.
Todo o produto não utilizado e o material de desperdício devem ser eliminados de acordo com os requisitos locais.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição deFator IX Grifols
O princípio ativo é fator IX de coagulação humano.
Cada frasco de Fator IX Grifols contém pó liofilizado com 250 UI, 500 UI, 1000 UI ou 1500 UI de fator IX de coagulação humano. Uma vez reconstituído, o conteúdo de fator IX humano é de 50 UI/ml (250 UI/5 ml, 500 UI/10 ml, 1000 UI/20 ml ou 1500 UI/30 ml).
Os outros componentes são lisina, glicina, cloreto, sódio, fosfato e citrato.
Cada envase de dissolvente contém 5 ml, 10 ml, 20 ml ou 30 ml de água para injetáveis.
Ver seção 2 para informação importante sobre alguns dos componentes.
Aspecto do produto e conteúdo do envase
Frasco contendo pó branco ou amarelo pálido e frasco/seringa com água para injetáveis (dissolvente).
Cada frasco de Fator IX Grifols das apresentações de 250 UI/5 ml, 500 UI/10 ml e 1000 UI/20 ml vai acompanhado de uma seringa pré-carregada de dissolvente que contém 5 ml, 10 ml ou 20 ml de água para injetáveis, juntamente com os acessórios necessários para sua reconstituição e administração (adaptador de frasco, filtro, 2 toalhetas com álcool e agulha borboleta).
Cada frasco de Fator IX Grifols da apresentação de 1500 UI/30 ml vai acompanhado de um frasco de dissolvente com 30 ml de água para injetáveis, juntamente com os acessórios necessários para sua reconstituição e administração (agulha de dupla ponta, filtro, 2 toalhetas com álcool, agulha borboleta e seringa com agulha).
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Conteúdo da caixa: 1 frasco de liofilizado, 1 seringa pré-carregada/frasco de dissolvente e acessórios.
Título da autorização de comercialização e responsável pela fabricação
Instituto Grifols, S.A.
Can Guasch, 2 - Parets del Vallès
08150 Barcelona - ESPANHA
Data da última revisão deste prospecto: abril 2019
A informação detalhada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) (http://www.aemps.gob.es/).
……………………………………………………………………………………………………………
Esta informação está destinada unicamente a profissionais do setor sanitário:
Pode ser empregada a seguinte tabela como guia de dosificação em episódios hemorrágicos e de cirurgia.
Grau da hemorragia/ Tipo de cirurgia | Nível de fator IX requerido (%)(UI/dl) | Frequência de dosificação (horas)/ Duração da terapia (dias) |
Hemorragia | ||
Hemartrose e sangramento muscular ou oral menores | 20 – 40 | Repetir cada 24 horas. Pelo menos 1 dia, até que o episódio hemorrágico manifestado por dor se detenha ou até curação. |
Hemartrose e hemorragia muscular ou hematoma moderados | 30 – 60 | Repetir a infusão cada 24 horas durante 3 – 4 dias ou mais até que a dor e a discapacidade aguda desapareçam. |
Hemorragias com perigo para a vida | 60 – 100 | Repetir a infusão cada 8 – 24 horas até que o risco desapareça. |
Cirurgia | ||
Cirurgia menor incluindo extrações dentárias Cirurgia maior | 30 – 60 80 – 100 (pré- e pós-operatório) | Cada 24 horas, pelo menos 1 dia até curação. Repetir a infusão cada 8 – 24 horas até a adequada cicatrização da ferida, e luego tratamento durante um mínimo de 7 dias para manter um nível de atividade de fator IX de 30% a 60% (UI/dl). |
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVELForma farmacêutica: INJETÁVEL, 1.000 UISubstância ativa: coagulation factor IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requer receita médicaForma farmacêutica: INJETÁVEL, 2.000 UISubstância ativa: coagulation factor IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requer receita médicaForma farmacêutica: INJETÁVEL, 250 UISubstância ativa: coagulation factor IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requer receita médica
Alternativas a FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL em Poland
Alternativa a FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL em Ukraine
Médicos online para FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de FACTOR IX GRIFOLS 50 UI/ml PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL – sujeita a avaliação médica e regras locais.














