Como usar ENTYVIO 108 mg SOLUÇÃO INJETÁVEL EM CANETA PREENCHIDA
Introdução
Entyvio 108mg solução injetável em caneta pré-carregada
vedolizumab
Leia todo o folheto detenidamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este folheto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste folheto. Ver secção 4.
Conteúdo do folheto
- O que é Entyvio e para que é utilizado
- O que precisa saber antes de começar a usar Entyvio
- Como usar Entyvio
- Efeitos adversos possíveis
- Conservação de Entyvio
- Conteúdo do envase e informações adicionais
1. O que é Entyvio e para que é utilizado
O que é Entyvio
Entyvio contém o princípio ativo “vedolizumab”. Vedolizumab pertence a um grupo de medicamentos biológicos denominados anticorpos monoclonais (Mab).
Como funciona Entyvio
Entyvio bloqueia uma proteína na superfície dos glóbulos brancos (leucócitos) que causa a inflamação na colite ulcerosa e na doença de Crohn, de modo que se reduz a inflamação.
Para que é indicado Entyvio
Entyvio é utilizado para tratar os sinais e sintomas em adultos com:
- colite ulcerosa ativa de moderada a grave
- doença de Crohn ativa de moderada a grave.
Colite ulcerosa
A colite ulcerosa é uma doença que provoca a inflamação do intestino grosso. Se padece colite ulcerosa, serão administrados primeiro outros medicamentos. Se não responder de maneira satisfatória ou não tolerar ditos medicamentos, o seu médico pode prescrever-lhe Entyvio para reduzir os sinais e sintomas da doença.
Doença de Crohn
A doença de Crohn é uma doença que provoca a inflamação do aparelho digestivo. Se padece doença de Crohn, serão administrados primeiro outros medicamentos. Se não responder de maneira satisfatória ou não tolerar ditos medicamentos, o seu médico pode prescrever-lhe Entyvio para reduzir os sinais e sintomas da doença.
2. O que precisa saber antes de começar a usar Entyvio
Não use Entyvio
- se é alérgico a vedolizumab ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
- se tem uma infecção ativa grave, por exemplo, tuberculose, septicemia, vómitos ou diarreia graves (gastroenterite) ou infecção do sistema nervoso.
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de usar Entyvio.
Informar o seu médico, farmacêutico ou enfermeiro imediatamentequando use este medicamento pela primeira vez, durante o tratamento e nos períodos entre doses:
- se experimentar visão dupla, borrosa ou perda de visão, dificuldade na fala, fraqueza em um braço ou perna, um cambio no seu modo de caminhar ou problemas de equilíbrio, entorpecimento persistente, diminuição ou perda de sensibilidade, confusão ou perda de memória. Todos estes sintomas poderiam corresponder a uma complicação cerebral grave e potencialmente mortalconhecida como leucoencefalopatia multifocal progressiva (LMP).
- se tem uma infecção, ou acredita que tem uma infecção – os sinais podem ser calafrios, tremores, tos persistente ou febre alta –. Algumas infecções podem ser graves e até potencialmente mortais se não forem tratadas.
- se experimentar sinais de uma reação alérgicacomo silvos, dificuldade para respirar, habones, picos, inchação ou tonturas. Para informações mais detalhadas, consulte o apartado sobre reações alérgicas na secção 4.
- se vai receber alguma vacinaou a recebeu recentemente. Entyvio pode afetar a forma como você responde a uma vacina.
- se tem cancro, diga ao seu médico. O seu médico terá que decidir se é possível administrar-lhe Entyvio.
- se não se sente nada melhor, dado que o vedolizumab pode tardar até 14 semanas em actuar em alguns doentes com doença de Crohn muito ativa.
Crianças e adolescentes
Entyvio não é recomendado para uso em crianças e adolescentes (menores de 18 anos de idade) devido à falta de informações sobre a utilização deste medicamento neste grupo de idade.
Outros medicamentos e Entyvio
Informar o seu médico, farmacêutico ou enfermeiro se está a tomar, tomou recentemente ou pudesse ter que tomar qualquer outro medicamento.
- Entyvio não deve ser administrado com outros medicamentos biológicos supressores do sistema imunológico, porque os efeitos que se podem produzir são desconhecidos.
Informar o seu médico se anteriormente lhe foi administrado:
- natalizumab (um medicamento indicado para a esclerose múltipla) ou
- rituximab (um medicamento indicado para certos tipos de cancro e a artrite reumatoide).
O seu médico terá que decidir se é possível administrar-lhe Entyvio.
Gravidez e amamentação
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento.
Gravidez
Os efeitos de Entyvio em mulheres grávidas são desconhecidos. Por isso, não se recomenda utilizar este medicamento durante a gravidez. Você e o seu médico devem decidir que o benefício para si é claramente superior ao risco potencial para si e o seu bebê.
Se é uma mulher em idade fértil, recomenda-se que evite engravidar durante o uso de Entyvio. Deve utilizar métodos anticonceptivos adequados durante o tratamento e durante pelo menos 4,5 meses após receber a última dose.
Amamentação
Informar o seu médico se está em período de amamentação ou tem intenção de estar. Entyvio passa para o leite materno. Não existe informação suficiente relativa aos efeitos que isso pode ter no seu bebê e na produção de leite. Deve decidir-se se interromper a amamentação ou o tratamento com Entyvio, para o que será necessário avaliar os benefícios da amamentação para o seu bebê e os benefícios do tratamento para si.
Condução e uso de máquinas
Os efeitos deste medicamento sobre a capacidade para conduzir e utilizar máquinas ou ferramentas são pequenos. Um número reduzido de doentes se sentiram tontos após receber Entyvio. Se se sentir tonto, não conduza nem utilize ferramentas ou máquinas.
Entyvio 108mg solução injetável contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por unidade de dose; isto é, é essencialmente “exento de sódio”.
3. Como usar Entyvio
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte-os de novo.
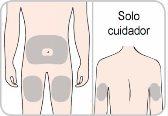
Vous ou o seu cuidador receberão formação sobre o uso das injeções sob a pele (as injeções subcutâneas) de Entyvio.
Quanto Entyvio será administrado
O tratamento com Entyvio é o mesmo para a colite ulcerosa e para a doença de Crohn.
A dose recomendada é de 108 mg de Entyvio administrados mediante injeção subcutânea uma vez cada 2 semanas.
- No início do tratamento, o seu médico lhe administrará as doses iniciais de Entyvio mediante um sistema de perfusão com goteo em uma das veias do braço (perfusão intravenosa) durante cerca de 30 minutos.
- Após pelo menos 2 perfusões intravenosas, poderá iniciar o tratamento com Entyvio mediante injeção subcutânea. A primeira injeção subcutânea é administrada no momento da próxima perfusão intravenosa programada, e cada 2 semanas a partir de então.
Como injetar Entyvio
Vous mesmo pode administrar as injeções subcutâneas, ou pode fazê-lo um cuidador, após receber formação sobre isso. No final deste folheto, encontrará instruções sobre como proceder à injeção subcutânea.
Se esqueceu ou omitiu uma injeção de Entyvio
Se esqueceu ou omitiu uma dose, injete a próxima dose assim que possível e, a seguir, cada 2 semanas.
Se interromper o tratamento com Entyvio
Não deve deixar de usar Entyvio sem falar antes com o seu médico.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Efeitos adversos possíveis
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Efeitos adversos graves
Informar o seu médico imediatamentese notar algum dos seguintes sintomas:
- reações alérgicas (podem afetar até 1 de cada 100 doentes), com sinais como silvos ou dificuldade para respirar, habones, picos, inchação, sensação de mal-estar, dor no local de perfusão, eritema
- infecções (podem afetar até 1 de cada 10 doentes), com sinais como calafrios ou tremores, febre alta ou erupções
Outros efeitos adversos
Informar o seu médico o mais breve possívelse notar algum dos seguintes sintomas:
Efeitos adversos muito frequentes(podem afetar mais de 1 de cada 10 doentes)
- resfriado comum
- dor nas articulações
- dor de cabeça
Efeitos adversos frequentes(podem afetar até 1 de cada 10 doentes)
- neumonia
- infecção bacteriana do intestino grosso por Clostridium difficile
- febre
- infecção respiratória
- mudanças no funcionamento do fígado, aumento das enzimas hepáticas (mostram-se nos análises de sangue)
- cansaço
- tosse
- gripe
- dor nas costas
- dor de garganta
- sinusite
- picos/prurido
- erupção e inchação
- dor nas extremidades
- cãibras musculares
- fraqueza muscular
- infecção de garganta
- gripe estomacal
- infecção anal
- dor anal
- fezes duras
- estômago inchado
- flatulências
- pressão arterial alta
- entorpecimento ou formigamento
- ardor de estômago
- hemorroides
- nariz entupido
- eczema
- sudorese noturna
- acne (espinhas)
- reações na zona de injeção (dor, inchação, eritema ou picos)
- herpes zóster
Efeitos adversos pouco frequentes(podem afetar até 1 de cada 100 doentes)
- inchação e sensibilidade dos folículos pilosos
- infecção de boca e garganta por leveduras
- infecção vaginal
- visão borrosa (perda da acuidade visual)
Efeitos adversos muito raros(podem afetar até 1 de cada 10.000 doentes)
- reação alérgica repentina e grave que pode causar dificuldade para respirar, inchação, aceleração do ritmo cardíaco, sudorese, queda da pressão arterial, tontura, perda de consciência e desmaio (reação anafiláctica e choque anafiláctico)
- inflamação do fígado (hepatite). Os sinais e sintomas de hepatite podem incluir provas de função hepática anormais, amarelamento dos olhos ou da pele (icterícia), dor na parte direita da área do estômago ou cardenales
Frequência não conhecida(a frequência não pode ser estimada a partir dos dados disponíveis)
- doença pulmonar que provoca dificuldade para respirar (doença pulmonar intersticial)
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste folheto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Entyvio
- Manter este medicamento fora da vista e do alcance das crianças.
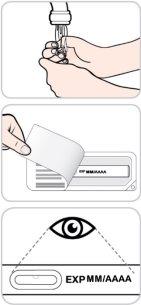
- Não utilize este medicamento após a data de validade que aparece no estojo e na etiqueta, após "CAD". A data de validade é o último dia do mês que se indica.
- Entyvio é para uso único.
- Conservar na geladeira (entre 2 °C e 8 °C). Conservar a(s) caneta(s) pré-carregada(s) no estojo original para protegê-las da luz. Se for necessário, uma única caneta pré-carregada pode ser conservada fora da geladeira, protegida da luz, a temperatura ambiente (até 25 °C) durante 7 dias, no máximo. Não utilize a caneta se levou mais de 7 dias fora da geladeira.
- Não congelar. Não exponha directamente à luz do sol.
- Não utilizar este medicamento se se observar presença de partículas no líquido ou decoloração (deve ser incolor ou apresentar uma tonalidade amarelada) antes da administração.
- Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Entyvio
- O princípio ativoé vedolizumab. Cada caneta precarregada contém 108 mg de vedolizumab.
- Os outros componentessão ácido cítrico monohidratado, citrato de sódio dihidratado, L-histidina, monohidrocloruro de L-histidina, hidrocloruro de L-arginina, polissorbato 80 e água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
- Entyvio é uma solução injetável incolor ou de cor amarela que é fornecida em uma caneta precarregada de vidro com um mecanismo de segurança automático que protege e bloqueia a agulha após a extração do dispositivo da zona de injeção.
- Entyvio está disponível em estuches com 1 ou 2 canetas precarregadas e em embalagens múltiplas com 6 (6 x 1) canetas precarregadas. Pode ser que apenas alguns tamanhos de embalagens sejam comercializados.
Título da autorização de comercialização
Takeda Pharma A/S
Delta Park 45
2665 Vallensbaek Strand
Dinamarca
Responsável pela fabricação
Takeda Austria GmbH
St. Peter-Straße 25
A-4020 Linz
Áustria
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica Takeda Belgium NV Tel.: +32 2 464 06 11 | Lituânia Takeda, UAB Tel.: +370 521 09 070 |
| Luxemburgo Takeda Belgium NV Tel.: +32 2 464 06 11 |
República Tcheca Takeda Pharmaceuticals Czech Republic s.r.o. Tel: +420 234 722 722 | Hungria Takeda Pharma Kft. Tel.: +36 1 2707030 |
Dinamarca Takeda Pharma A/S Tlf: +45 46 77 10 10 | Malta Drugsales Ltd Tel.: +356 2141 9070 |
Alemanha Takeda GmbH Tel.: +49 (0) 800 825 3325 | Países Baixos Takeda Nederland B.V. Tel.: +31 20 203 5492 |
Estônia Takeda Pharma OÜ Tel.: +372 6177 669 | Noruega Takeda AS Tlf.: +47 800 800 30 |
Grécia TAKEDA ΕΛΛΑΣ Α.Ε. Τηλ.: +30 210 6387800 | Áustria Takeda Pharma Ges.m.b.H. Tel.: +43 (0) 800 20 80 50 |
Espanha Takeda Farmacéutica España, S.A. Tel.: +34 917 90 42 22 | Polônia Takeda Pharma Sp. z o.o. Tel.: +48 223 062 447 |
França Takeda France SAS Tel.: +33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel.: +351 21 120 1457 |
Croácia Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | Romênia Takeda Pharmaceuticals SRL Tel.: +40 21 335 03 91 |
Irlanda Takeda Products Ireland Ltd. Tel.: 1800 937 970 | Eslovênia Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel.: +386 (0) 59 082 480 |
Islândia Vistor hf. Simi: +354 535 7000 | Eslováquia Takeda Pharmaceuticals Slovakia s.r.o. Tel.: +421 (2) 20 602 600 |
Itália Takeda Italia S.p.A Tel.: +39 06 502601 | Finlândia Takeda Oy Puh./Tel.: 0800 774 051 |
Chipre A.POTAMITIS MEDICARE LTD Τηλ: +357 22583333 | Suécia Takeda Pharma AB Tel.: 020 795 079 |
Letônia Takeda Latvia SIA Tel.: +371 67840082 | Reino Unido (Irlanda do Norte) Takeda UK Ltd Tel.: +44 (0) 3333 000 181 |
Data da última revisão deste prospecto: 03/2025
Outras fontes de informação
Este prospecto está disponível em formato adequado para pacientes cegos ou com visão reduzida e pode ser solicitado ao representante local do titular da autorização de comercialização.
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
-----------------------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a profissionais do setor sanitário:
Rastreabilidade
Com o objetivo de melhorar a rastreabilidade dos medicamentos biológicos, o nome e o número do lote do medicamento administrado devem estar claramente registrados.
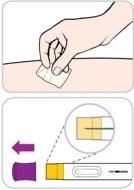
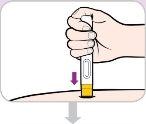
Instruções de uso:
Leia e siga estas instruções antes de proceder à injeção. Seu médico, enfermeiro ou farmacêutico devem mostrar-lhe como usar a caneta precarregada de Entyvio antes de usá-la pela primeira vez.
Sua caneta precarregada de Entyvio de uso único
Antes de usar | |||
Tampa de cor roxa | Janela de visualização | ||
|
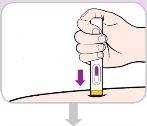
Depois de usar | |||
Proteção de agulha de cor amarela | Janela de visualização (injeção finalizada) | ||
|
| |
| Aguarde 30minutos
|
| |
|
|
| |
|
|
| |
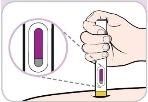
| PRESSIONE
SEGURE (contar até 10)
CONFIRME
|
| |
|
|
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a ENTYVIO 108 mg SOLUÇÃO INJETÁVEL EM CANETA PREENCHIDAForma farmacêutica: INJETÁVEL, 108 mgSubstância ativa: vedolizumabFabricante: Takeda Pharma A/SRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 300 mgSubstância ativa: vedolizumabFabricante: Takeda Pharma A/SRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 120 mg (80 mg/kg) belimumabeSubstância ativa: belimumabFabricante: Glaxosmithkline (Ireland) LimitedRequer receita médica
Médicos online para ENTYVIO 108 mg SOLUÇÃO INJETÁVEL EM CANETA PREENCHIDA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de ENTYVIO 108 mg SOLUÇÃO INJETÁVEL EM CANETA PREENCHIDA – sujeita a avaliação médica e regras locais.