
CLUVOT 250 UI, PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL OU PERFUSÃO

Pergunte a um médico sobre a prescrição de CLUVOT 250 UI, PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL OU PERFUSÃO

Como usar CLUVOT 250 UI, PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL OU PERFUSÃO
Introdução
Prospecto: informação para o utilizador
Cluvot 250 UI
Pó e dissolvente para solução injetável ou perfusão.
Concentrado de fator XIII de coagulação derivado de plasma humano
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
|
Conteúdo do prospecto
- O que é Cluvot e para que é utilizado
- O que precisa saber antes de começar a usar Cluvot
- Como usar Cluvot
- Posíveis efeitos adversos
- Conservação de Cluvot
- Conteúdo do envase e informação adicional
1. O que é Cluvot e para que é utilizado
O que é Cluvot
Cluvot apresenta-se como um pó branco e um dissolvente. A solução obtida deve ser administrada mediante injeção em uma veia.
Cluvot é um produto composto por fator XIII (FXIII) de coagulação derivado de plasma humano (a parte líquida do sangue), e tem funções importantes na hemostasia (interrupção da hemorragia).
Para que é utilizado Cluvot
Cluvot é utilizado em pacientes adultos e pediátricos
- para o tratamento preventivo da deficiência hereditária do fator XIII e
- para o tratamento perioperatório da hemorragia cirúrgica com deficiência congênita de FXIII
.
2. O que precisa saber antes de começar a usar Cluvot
As seções seguintes contêm informações que o seu médico deve ter em conta antes de lhe administrar Cluvot.
Não use Cluvot:
- se é alérgico ao princípio ativo ou a qualquer um dos outros componentes deste medicamento (incluídos na seção 6).
Informa ao seu médico se é alérgico a qualquer medicamento ou alimento.
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Cluvot.
- Se experimentou reações alérgicas ao FXIII de coagulação no passado. Deve tomar antihistamínicos e corticosteroides com fins profiláticos se assim o aconselhar o seu médico.
- Quando se produzirem reações de tipo alérgico ou anafiláctico (uma reação alérgica grave que provoca dificuldades respiratórias graves ou mareios), a administração de Cluvot deve ser interrompida de imediato (p. ex., interromper a perfusão). Em caso de choque, seguir-se-ão as orientações médicas vigentes para o seu tratamento.
- Se experimentou uma trombose recente (coágulo sanguíneo), deve agir com precaução devido ao efeito estabilizador da fibrina do FXIII.
- A formação de inibidores (anticorpos neutralizantes) é uma complicação conhecida do tratamento e implica que o tratamento deixou de funcionar. Se as suas hemorragias não conseguem ser controladas com Cluvot, informe o seu médico imediatamente. Deve ser controlado cuidadosamente por si desenvolver algum inibidor.
O seu médico considerará detidamente o benefício do tratamento com Cluvot em comparação com o risco dessas complicações.
Segurança viral
Quando os medicamentos são elaborados a partir de sangue ou plasma humano, são tomadas certas medidas para prevenir a transmissão de infecções aos pacientes. Estas medidas incluem:
- seleção cuidadosa dos doadores de sangue e plasma para garantir que se exclua os possíveis portadores de infecções;
- análise de cada doação e mistura de plasmas para detectar sinais de vírus/infecções;
- a inclusão de medidas durante o processamento do sangue ou plasma para inativar ou eliminar os vírus.
Apesar dessas medidas, quando se administram medicamentos preparados a partir de sangue ou plasma humano, não pode ser excluída totally a possibilidade de transmissão de infecções. Isso também se aplica a qualquer vírus desconhecido ou emergente ou a outros tipos de infecções.
As medidas adotadas são consideradas eficazes para os vírus envoltos como o vírus da imunodeficiência humana (VIH), o vírus da hepatite B e o vírus da hepatite C, e para o vírus não envolto da hepatite A e o parvovirus B19.
Recomenda-se encarecidamente que, cada vez que lhe for administrado Cluvot, o seu médico anote o nome e o número do lote do medicamento (encontra-se na caixa de cartão).
É possível que o seu médico lhe recomende que considere a possibilidade de se vacinar contra a hepatite A e B se receber de forma regular ou repetida produtos derivados de plasma humano.
Uso de Cluvot com outros medicamentos
- Informa ao seu médico ou farmacêutico se está utilizando, utilizou recentemente ou poderia ter que utilizar qualquer outro medicamento.
- Até o momento, não se conhece nenhuma interação entre o concentrado do FXIII de coagulação derivado de plasma humano e outros medicamentos.
- Cluvot não deve ser misturado com outros medicamentos, diluentes ou dissolventes, excepto aqueles indicados na seção 6 e deve ser administrado através de uma linha de perfusão independente.
Gravidez, lactação e fertilidade
- Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de que lhe seja administrado este medicamento.
- Os dados limitados sobre o uso clínico de Cluvot na gravidez não mostraram efeitos negativos na evolução da gravidez e no desenvolvimento perinatal ou pós-natal. Por tanto, se for necessário, pode ser considerado o uso de Cluvot durante a gravidez.
- Não há dados sobre a excreção de Cluvot no leite humano. No entanto, com base no seu grande tamanho molecular, a excreção no leite é improvável e, devido à sua natureza proteica, também é improvável a absorção de moléculas intactas pelo lactente. Por tanto, Cluvot pode ser utilizado durante a lactação.
- Não se dispõe de dados de fertilidade.
Condução e uso de máquinas
Não se realizaram estudos sobre os efeitos na capacidade para conduzir e utilizar máquinas.
Cluvot contém sódio
Tenha em conta que Cluvot contém sódio. Isso é importante se está a seguir uma dieta com restrição de sódio. Cluvot contém de 124,4 a 195,4 mg (de 5,41 a 8,50 mmol) de sódio por dose (40 UI/peso corporal: para uma média de 70 kg), se for aplicada a dose recomendada (2.800 UI = 44,8 ml).
3. Como usar Cluvot
- Normalmente o seu médico será quem lhe administrará Cluvot.
- Cluvot está destinado apenas para injeção venosa.
Dosagem
O seu médico calculará a dose correta e decidirá a frequência com que lhe será administrado Cluvot, tendo em conta como está funcionando o tratamento.
Para mais informações, ver seção “Esta informação está destinada apenas a profissionais do sector sanitário”.
Sobredosagem
Não se notificaram casos de sobredosagem e não se prevêem, dado que o medicamento é administrado por profissionais de saúde.
4. Posíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram.
Foram observados os seguintes efeitos adversos em raras ocasiões(afeta mais de 1 utilizador em cada 10.000 e menos de 1 utilizador em cada 1.000):
- Reações alérgicas, como a urticária generalizada (inchaços com picar na pele), erupção cutânea, descida da pressão arterial (que pode provocar sensação de desmaio ou mareio) e dificuldade para respirar.
- Aumento na temperatura
Foram observados os seguintes efeitos adversos em muito raras ocasiões(afeta menos de 1 utilizador em cada 10.000):
- Desenvolvimento de inibidores do FXIII.
Se se produzirem reações alérgicas, deve-se interromper de imediato a administração de Cluvot e iniciar o tratamento apropriado. Para o tratamento do choque, deve-se agir de acordo com as orientações médicas vigentes.
Efeitos adversos em crianças e adolescentes
Espera-se que os efeitos adversos em crianças sejam os mesmos que em adultos.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Cluvot
- Conservar em frigorífico (entre + 2 °C e + 8 °C).
- Não congelar.
- Conservar o frasco no envase exterior para protegê-lo da luz.
- Cluvot não contém conservantes. O produto deve ser utilizado de imediato após a reconstituição. Em caso de não ser utilizado de imediato, não deve ser conservado mais de 4 horas a temperatura ambiente. Não refrigerar nem congelar a solução reconstituída.
- Mantenha este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece na etiqueta e no envase de cartão. A data de validade é o último dia do mês que se indica.
- Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Cluvot
O princípio ativo é:
Concentrado de fator XIII de coagulação derivado de plasma humano que contém 250 UI por frasco.
Os outros componentes são:
Albumina humana, glicose monohidratada, cloreto de sódio, hidróxido de sódio (em pequenas quantidades para o ajuste do pH)
Diluente:Água para preparações injetáveis
Aspecto do produto e conteúdo do envase
Cluvot apresenta-se como um pó branco e é fornecido com um diluente (água para preparações injetáveis).
A solução obtida deve ser incolor, transparente ou ligeiramente opalescente. Quando exposta à luz, não deve ser turva nem conter resíduos (depósitos/partículas).
Apresentação
Um envase de 250 UI que contém:
- 1 frasco com pó
- 1 frasco com 4 ml de água para preparações injetáveis
- 1 trasvasador com filtro 20/20 (Mix2Vial)
- Equipamento de administração (caixa interior):
- 1 seringa de 5 ml descartável
- 1 equipamento de venopunção
- 2 compressas impregnadas de álcool
- 1 curativo não estéril
Título da autorização de comercialização e responsável pela fabricação
CSL Behring GmbH
Emil-von-Behring-Strasse 76
35041 Marburg (Alemanha)
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
CSL Behring, S.A.
c/ Tarragona 157, planta 18
08014 Barcelona (Espanha)
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:Cluvot
Data da última revisão deste prospecto: outubro 2018.
A informação detalhada e atualizada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es
_____________________________________________________________________________________
Esta informação está destinada unicamente a profissionais do sector sanitário:
Posologia e forma de administração
Posologia
1 ml equivale aproximadamente a 62,5 UI, e 100 UI equivalem a 1,6 ml, respetivamente.
Importante:
A quantidade que deve ser administrada e a frequência de administração sempre devem orientar-se pela eficácia clínica em cada caso particular.
Dosagem
A pauta de dosagem deve ser personalizada de acordo com o peso corporal, os valores analíticos e o estado clínico do paciente.
Pauta de dosagem profilática habitual
Dose inicial
- 40 unidades internacionais (UI) por kg de peso corporal
- A velocidade de injeção não deve ser superior a 4 ml por minuto.
Dosagem posterior
- A dosagem deve guiar-se pelo nível de atividade mínimo mais recente do FXIII, com uma administração cada 28 dias (4 semanas) para manter um nível de atividade mínimo do FXIII entre 5 e 20%, aproximadamente.
- Os ajustes de dose recomendados de ± 5 UI por kg devem basear-se nos níveis de atividade mínimos do FXIII tal como se mostra na tabela 1 e no estado clínico do paciente.
- Os ajustes de dose devem realizar-se baseando-se num análise específico e sensível para determinar os níveis de FXIII. Na tabela 1 seguinte, descreve-se um exemplo de ajuste da dose mediante a prova de atividade Berichrom® padrão.
Tabela 1: ajuste da dose mediante a prova de atividade Berichrom®
Nível mínimo de atividade do fator XIII (%) | Mudança de dosagem |
Um nível mínimo de < 5% | Aumento em 5 unidades por kg |
Nível mínimo entre 5% e 20% | Sem mudança |
Dois níveis mínimos de > 20% | Diminuição em 5 unidades por kg |
Um nível mínimo de > 25% | Diminuição em 5 unidades por kg |
A potência expressa em unidades determina-se mediante a prova de atividade Berichrom®, referida ao padrão internacional vigente para o fator XIII de coagulação sanguínea no plasma.
Portanto, uma unidade neste documento é equivalente a uma unidade internacional.
Profilaxia prévia à cirurgia
Depois da última dose profilática habitual do paciente, se uma cirurgia for programada:
- Entre 21 e 28 dias depois: administre ao paciente a dose profilática total imediatamente antes da cirurgia e a próxima dose profilática deve ser administrada 28 dias depois.
- Entre 8 e 21 dias depois: pode ser administrada uma dose adicional (total ou parcial) antes da cirurgia. A dose deve guiar-se pelos níveis de atividade do FXIII e pelo estado clínico do paciente e deve ajustar-se de acordo com a meia-vida de Cluvot.
- Nos 7 dias posteriores desde a última dose: pode não ser necessária uma dose adicional.
Os ajustes de dose podem diferir dessas recomendações e devem personalizar-se em função dos níveis de atividade do FXIII e do estado clínico do paciente. Deve controlar-se com precisão todos os pacientes durante e após a cirurgia.
Portanto, recomenda-se monitorizar o aumento da atividade do FXIII com uma prova de FXIII. Em caso de cirurgia maior e hemorragias graves, o objetivo é obter valores quase normais (pessoas saudáveis: 70% - 140%).
População pediátrica
A posologia e forma de administração em crianças e adolescentes baseiam-se no peso corporal e, portanto, em geral baseiam-se nas mesmas diretrizes que para os adultos. A dose e a frequência de administração para cada pessoa sempre devem guiar-se pela eficácia clínica e pelos níveis de atividade do fator XIII.
População de idade avançada
A posologia e forma de administração em pessoas de idade avançada (> 65 anos) não se documentou ainda em estudos clínicos.
Forma de administração
Instruções gerais
- A solução deve ser transparente ou ligeiramente opalescente. Depois de extrair e filtrar o produto reconstituído (ver mais adiante) deve revisar-se visualmente para detectar a presença de partículas estranhas e decolorações antes da administração.
- Não se devem utilizar soluções visivelmente turvas ou soluções que ainda contenham flóculos ou partículas.
- A reconstituição e a extração devem realizar-se em condições assépticas.
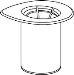
Reconstituição
Aquecer o diluente à temperatura ambiente. Certifique-se de que se tenham retirado as cápsulas dos frascos do produto e do diluente e de que os tampões sejam tratados com uma solução asséptica e se deixem secar antes de abrir o envase do Mix2Vial.
|
|
|
|
|
|
|
|
|
Elimine o frasco do diluente com o adaptador do Mix2Vial azul acoplado. |
|
|
|
|
Transvasamento e administração
|
puxando o êmbolo lentamente para trás. |
|
|
Deve ter-se cuidado para que não entre sangue na seringa cheia do produto, pois existe risco de que o sangue se coagule na seringa e, portanto, se administrem ao paciente coágulos de fibrina.
A solução reconstituída deve ser administrada por via intravenosaem uma linha de injeção/perfusão independente (fornecida com o produto) mediante uma injeção lenta, a uma velocidade que não exceda 4 ml por minuto.
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contacto com ele realizar-se-á de acordo com a normativa local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a CLUVOT 250 UI, PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL OU PERFUSÃOForma farmacêutica: INJETÁVEL, 1.000 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médicaForma farmacêutica: INJETÁVEL, 1500 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médicaForma farmacêutica: INJETÁVEL, 1000 UI - após reconstituição em 2 ml de água para injetáveis, a dose é de 500 UI/mlSubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médica
Alternativas a CLUVOT 250 UI, PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL OU PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a CLUVOT 250 UI, PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL OU PERFUSÃO em Polónia
Médicos online para CLUVOT 250 UI, PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL OU PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de CLUVOT 250 UI, PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL OU PERFUSÃO – sujeita a avaliação médica e regras locais.




 1
1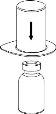 2
2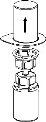 3
3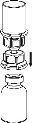 4
4 5
5 6
6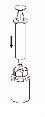 7
7 8
8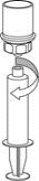 9
9









