BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEA

Pergunte a um médico sobre a prescrição de BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEA

Como usar BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEA
Introdução
Prospecto: informação para o utilizador
Berinert 3000 UI
Pó e diluente para solução injetável subcutânea
Inibidor da C1 esterase humano
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas para si e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, pois pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto:
- O que é Berinert e para que é utilizado
- O que precisa saber antes de começar a usar Berinert
- Como usar Berinert
- Possíveis efeitos adversos
- Conservação de Berinert
- Conteúdo do envase e informações adicionais
1. O que é Berinert e para que é utilizado
O que é Berinert?
Berinert apresenta-se como pó e diluente. A solução preparada deve ser administrada por injeção sob a pele.
Berinert é preparado a partir de plasma humano (a parte líquida do sangue). O princípio ativo é a proteína inibidora da C1 esterase humana, obtida a partir do plasma.
Para que é utilizado Berinert?
Berinert é utilizado para a prevenção de ataques recorrentes de angioedema hereditário (AEH) em pacientes adolescentes e adultos. O angioedema hereditário é uma doença congénita do sistema vascular. Não é uma doença alérgica. O AEH é causado por uma síntese insuficiente, ausente ou defeituosa do inibidor da C1 esterase, que é uma proteína importante. A doença caracteriza-se pelos sintomas seguintes:
- inchação súbita das mãos e pés,
- inchação súbita do rosto com sensação de tensão,
- inchação das pálpebras, lábios, possível inchação da laringe (órgão da voz) com dificuldades respiratórias,
- inchação da língua,
- dor de tipo cólico na região abdominal.
Geralmente, todas as partes do corpo podem ser afetadas.
2. O que precisa saber antes de começar a usar Berinert
As secções seguintes contêm informações que o seu médico deve ter em consideração antes de administrar-lhe Berinert.
Não use Berinert:
- Se experimentou reações de hipersensibilidade imediata de risco vital, incluindo anafilaxia, ao inibidor da C1 esterase ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
Informar o seu médico ou farmacêutico se é alérgico a algum medicamento ou a algum alimento.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a usar Berinert,
- se ocorrem reações alérgicas ou anafilácticas graves (reação alérgica grave que produz graves dificuldades respiratórias ou tonturas). A administração de Berinert deve ser interrompida imediatamente (por exemplo, interrompendo a injeção).
- Se apresenta um histórico de problemas de coagulação do sangue. Foram observados coágulos sanguíneos em pacientes tratados com Berinert por via intravenosa. O uso de Berinert a doses muito altas em doenças distintas do AEH pode aumentar o risco de coágulos de sangue. No entanto, no caso de Berinert por via subcutânea, não existe uma relação estabelecida com coágulos de sangue à dose que se recomenda que prescreva o seu médico. Consulte com o seu médico se apresenta um histórico de doença cardíaca ou dos vasos sanguíneos, infarto, coágulos sanguíneos ou sangue espessa, um catéter permanente/dispositivo de acesso em uma das suas veias ou esteve imobilizado durante algum tempo. Estas situações podem aumentar o risco de apresentar um coágulo após o uso de Berinert. Informe também o seu médico sobre os medicamentos que está utilizando, pois alguns medicamentos, como os anticoncepcionais ou certos andrógenos, podem aumentar o risco de desenvolver um coágulo sanguíneo.
O seu médico sopesará minuciosamente o benefício do tratamento com Berinert comparando com o risco de padecer estas complicações.
Segurança viral
Quando se administram medicamentos derivados do sangue ou plasma humanos, há que levar a cabo certas medidas para evitar que as infecções passem aos pacientes. Tais medidas incluem:
- seleção cuidadosa dos doadores de sangue e plasma para excluir aqueles que estão em risco de ser portadores de doenças infecciosas, e
- análise de marcadores específicos de vírus e infecções nas doações individuais e nas misturas de plasma.
Os fabricantes destes produtos também incluem etapas no processamento do sangue ou plasma para eliminar/inativar vírus. Apesar disto, quando se administram medicamentos derivados do sangue ou plasma humanos, a possibilidade de transmissão de agentes infecciosos não se pode excluir totalmente. Isto também se refere a vírus emergentes ou de natureza desconhecida ou outro tipo de infecções.
As medidas aplicadas consideram-se eficazes para os vírus envoltos, tais como o vírus de imunodeficiência humano (VIH, o vírus da SIDA), o vírus da hepatite B, o vírus da hepatite C (inflamação do fígado) e para vírus não envoltos, como o vírus da hepatite A (inflamação do fígado) e o parvovirus B19.
É possível que o seu médico lhe recomende a vacinação contra a hepatite A e B se se tratar periodicamente/repetidamente com medicamentos derivados do plasma humano.
Recomenda-se encarecidamente que cada vez que se administre Berinert, se registe a data de administração, o número do lote e o volume injetado.
Uso de Berinert com outros medicamentos
- Informar o seu médico ou farmacêutico se está utilizando, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento, mesmo os medicamentos sem prescrição.
- Berinert não se deve misturar com outros medicamentos e diluentes na mesma seringa.
Gravidez e lactação
- Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Condução e uso de máquinas
Berinert não afeta a sua capacidade de conduzir e de usar máquinas.
Berinert contém sódio
Este medicamento contém 29 mg de sódio (componente principal do sal de mesa/para cozinhar) em cada frasco. Isto equivale a 1,5% da ingestão diária máxima de sódio recomendada para um adulto.
3. Como usar Berinert
Berinert está indicado para a auto-administração por injeção subcutânea. O senhor ou o seu cuidador deve formar-se, tanto quanto seja necessário, sobre o modo de administrar Berinert.
Posologia
A dose recomendada de Berinert é de 60 UI/kg de peso.
População pediátrica
A dose recomendada é a mesma que em adultos.
Se usar mais Berinert do que deve
Não se descreveram casos de sobredosagem.
Reconstituição e forma de administração
Se o seu médico decidir que o senhor pode tratar-se em casa, este lhe dará instruções detalhadas. Ser-lhe-á entregue um diário onde anotará cada injeção administrada em casa, que o levará consigo cada vez que visitar o médico. Será revisto regularmente a si ou ao seu cuidador como administram as injeções para assegurar que o fazem corretamente ao longo do tempo.
Instruções gerais
- O pó deve dissolver-se e extrair-se do frasco sob condições assépticas. Utilize a seringa proporcionada com o produto.
- A solução preparada deve ser transparente e clara a ligeiramente opalescente. Depois de filtrar ou trasvasar a solução (consulte mais adiante), deve verificar-se visualmente que a solução não contenha partículas nem apresente decoloração antes de administrá-la.
- Não use a solução se estiver visivelmente turva ou se contiver partículas ou resíduos.
- Qualquer quantidade do medicamento que não se tenha usado ou qualquer material residual deve eliminar-se de acordo com a normativa local e seguindo as instruções do seu médico.
Reconstituição
Antes de abrir qualquer frasco, tempere o pó Berinert e o diluente até que estejam à temperatura ambiente. Para consegui-lo, pode deixar os frascos à temperatura ambiente durante aproximadamente uma hora ou bem pode tê-los nas mãos cerradas durante uns minutos. NÃO exponha os frascos ao calor direto. Os frascos não se devem aquecer a uma temperatura superior à do corpo (37 °C).
Retire com cuidado as cápsulas protectoras do frasco do diluente e do frasco com o pó. Limpe os tampões de borracha expostos de ambos os frascos com uma toalhita impregnada em álcool e deixe-os secar. Agora pode transferir o diluente para o frasco do pó com o sistema de administração incluído (Mix2Vial). Por favor, siga as instruções seguintes:
|
|
|
|
|
|
|
|
|
Elimine o frasco do diluente com o adaptador azul do Mix2Vial acoplado. |
|
|
|
|
Trasvase e administração
8 |
|
9 |
|
Administração
Auto-administração (administração subcutânea)
O seu médico ensinar-lhe-á como administrar Berinert de forma segura. Uma vez que já saiba como se auto-administrar o medicamento, siga as instruções que se proporcionam a seguir.
Tabela 2. Instruções para a auto-administração de Berinert
Paso 1: Acople os acessórios Tome a seringa Berinert, os seguintes acessórios descartáveis, e outros artigos (agulhas ou outros envases, diário de tratamento ou livro de registo):
| |
Paso 2: Limpe a superfície
| |
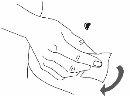
Paso 3: Lave as mãos
| |
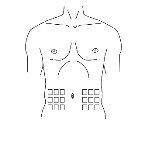
Paso 4: Prepare o ponto de injeção
|
Figura 1
Figura 2 |
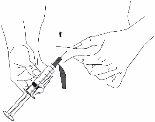
Paso 5: Injeção na zona abdominal Conforme lhe indique o seu profissional sanitário:
Injeção com agulha hipodérmica:
Injeção com equipamento de perfusão subcutânea:
|
Figura 3 |
Figura 4 | |
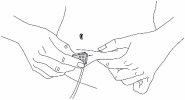
Paso 6: Limpe
| |
Paso 7: Registre o tratamento
|
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram.
Entre em contato imediatamente com o seu médico
- se produzir qualquer efeito adverso ou
- se notar qualquer efeito adverso não mencionado neste prospecto.
Os efeitos adversos com Berinert são raros.
Os seguintes efeitos adversos foram observados muito frequentemente (podem afetar mais de 1 de cada 10 pessoas):
- Reações no local onde se administrou a injeção (equimoses, sensação de frio, supuração, eritema, hematoma, hemorragia, endurecimento, edema, dor, prurido, sarpullido, cicatriz, inchação, urticária, calor).
- Nasofaringite (nariz congestionado, espirros, olhos lacrimejantes).
Os efeitos adversos seguintes foram observados frequentemente (podem afetar até 1 de cada 10 pessoas):
- Reações de hipersensibilidade ou alérgicas (como por exemplo hipersensibilidade, prurido, sarpullido e urticária)
- Tonturas
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Berinert
- Mantenha este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece na etiqueta e no envase de cartão após EXP.
- Não conserve a uma temperatura superior a 30 °C.
- Não congele.
- Conserva o frasco no embalagem exterior para protegê-lo da luz.
- Berinert não contém conservantes, de modo que é preferível que a solução preparada se utilize imediatamente.
- Se a solução preparada não se administra imediatamente, deve utilizar-se no prazo de 8 horas e apenas se deve conservar no frasco.
6. Conteúdo do envase e informação adicional
Composição de Berinert
O princípio ativo é:
Inibidor da C1 esterase humano (3.000 UI/frasco; após a reconstituição com 5,6 ml de água para preparações injetáveis 500 UI/ml).
Para mais informações, ver a seção “Estainformação está destinada unicamente a profissionais do sector sanitário”.
Os demais componentes são:
Glicina, cloreto de sódio, citrato de sódio.
Veículo:água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
Berinert apresenta-se como um pó branco e é fornecido com água para preparações injetáveis como veículo.
A solução preparada deve ser transparente e clara a ligeiramente opalescente.
Apresentação
Um envase contém:
1 frasco com pó
1 frasco com 5,6 ml de água para preparações injetáveis
1 trasvasador com filtro 20/20
Equipamento de administração (caixa interior):
1 seringa de 10 ml descartável
1 agulha hipodérmica
1 equipamento para injeção subcutânea (borboleta)
2 toalhetas com álcool
1 pensamento
Envase múltiplo de 5 x 3.000 UI, incluindo uma caixa com 5 equipamentos de administração.
Envase múltiplo de 20 x 3.000 UI, incluindo 4 caixas com 5 equipamentos de administração.
Pode ser que apenas alguns tamanhos de envase sejam comercializados.
Título da autorização de comercialização e responsável pela fabricação
CSL Behring GmbH
Emil-von-Behring-Strasse 76
35041 Marburg
Alemanha
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
CSL Behring S.A.
c/ Tarragona 157, planta 18
08014 Barcelona
Espanha
Este medicamento está autorizado nos estados membros do Espaço Económico Europeue no Reino Unido (Irlanda do Norte)com os seguintes nomes:
Berinert 3000 I.E. Pó e
veículo para solução injetável Áustria
Berinert 3000 IE, pó e veículo
para solução para injeção Bélgica, Países Baixos
Berinert 3000 Chipre, Alemanha, Grécia, Polônia, Portugal
???????? 3000, ???? ? ???????????
?? ??????????? ???????
C1- ????????? ?????????, ??????? Bulgária
Berinert 3000 IU República Checa, Eslováquia
Berinert 3000 IU pó e veículo
para solução para injeção _______________________________ Croácia
Berinert Dinamarca, Itália
Berinert SC Estônia
Berinert 3000 IU, pó para injeção
e veículo, solução para injeção Finlândia
Berinert 3000 UI, pó e veículo
para solução injetável França, Luxemburgo
Berinert 3000 NE pó e veículo
para solução injetável Hungria
Berinert 3000 a.e. pó e veículo
para solução para injeção Islândia
Human C1-esterase inhibitor CSL Behring
3000 UI pó e veículo para solução
injetável Lituânia
Berinert 3000 IU pó e veículo
para solução injetável Noruega
Berinert 3000 3000 UI, pó e veículo
para solução injetável Romênia
Berinert 3000 i.e. pó e veículo
para solução para injeção Eslovênia
Berinert 3000 UI pó e veículo
para solução injetável subcutânea Espanha
Berinert 3000 IE, pó e veículo
para solução injetável Suécia
Berinert 3000 IU pó e veículo
para solução para injeção _____________________ Reino Unido, Malta, Irlanda
Data da última revisão deste prospecto:Outubro 2021
A informação detalhada e atualizada deste medicamento está disponível no site da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es
Estainformação está destinada unicamente a profissionais do sector sanitário
COMPOSIÇÃO QUALITATIVA E QUANTITATIVA
A potência do inibidor da C1 esterase humano é expressa em Unidades Internacionais (UI), que está relacionada com o padrão atual da OMS para os produtos inibidores da C1 esterase.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEAForma farmacêutica: INJETÁVEL, 1500 UISubstância ativa: c1-inhibitor, plasma derivedFabricante: Csl Behring GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 2000 UISubstância ativa: c1-inhibitor, plasma derivedFabricante: Csl Behring GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 500 USubstância ativa: c1-inhibitor, plasma derivedFabricante: Csl Behring GmbhRequer receita médica
Alternativas a BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEA noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEA em Poland
Alternativa a BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEA em Ukraine
Médicos online para BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de BERINERT 3000 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL SUBCUTÂNEA – sujeita a avaliação médica e regras locais.




 1
1 2
2 3
3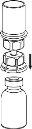 4
4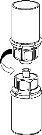 5
5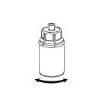 6
6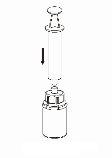 7
7