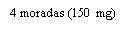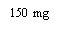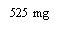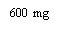XOLAIR 150 MG SOLUCION INYECTABLE

Cómo usar XOLAIR 150 MG SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Xolair 150mg solución inyectable en jeringa precargada
(jeringa precargada con aguja fijada de calibre 26, protector de la jeringa morado)
omalizumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Xolair y para qué se utiliza
- Qué necesita saber antes de empezar a usar Xolair
- Cómo usar Xolair
- Posibles efectos adversos
- Conservación de Xolair
- Contenido del envase e información adicional
1. Qué es Xolair y para qué se utiliza
Xolair contiene la sustancia activa omalizumab. Omalizumab es una proteína humana, similar a las proteínas naturales producidas por el organismo. Pertenece a una clase de medicamentos denominados anticuerpos monoclonales.
Xolair se utiliza para el tratamiento de:
- asma alérgica
- rinosinusitis crónica (inflamación de la nariz y de los senos) con pólipos nasales
- urticaria crónica espontánea (UCE)
Asma alérgica
Este medicamento se utiliza para prevenir que el asma empeore controlando los síntomas del asma alérgica grave en adultos, adolescentes y niños (a partir de 6 años de edad) que ya están recibiendo medicamentos para el asma, pero cuyos síntomas no se controlan adecuadamente con medicamentos tales como esteroides inhalados a dosis altas y beta agonistas inhalados.
Rinosinusitis crónica con pólipos nasales
Este medicamento se utiliza para tratar la rinosinusitis crónica con pólipos nasales en adultos (a partir de 18 años de edad) que están recibiendo corticosteroides intranasales (pulverización nasal con corticosteroides), pero cuyos síntomas no están bien controlados con estos medicamentos. Los pólipos nasales son pequeños crecimientos en el revestimiento de la nariz. Xolair ayuda a reducir el tamaño de los pólipos y mejora los síntomas incluyendo la congestión nasal, pérdida del sentido del olfato, mucosidad en la parte posterior de la garganta y secreción nasal.
Urticaria crónica espontánea (UCE)
Este medicamento se utiliza para el tratamiento de la urticaria crónica espontánea en adultos y adolescentes (a partir de 12 años de edad) que ya están recibiendo antihistamínicos pero cuyos síntomas de la UCE no están bien controlados por estos medicamentos.
Xolair actúa bloqueando una sustancia llamada inmunoglobulina E (IgE) que es producida por el organismo. La IgE interviene en un tipo de inflamación que juega un papel clave como causante del asma alérgica, la rinosinusitis crónica con pólipos nasales y UCE.
2. Qué necesita saber antes de empezar a usar Xolair
No use Xolair
- si es alérgico a omalizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Si cree que puede ser alérgico a cualquiera de los componentes, informe a su médico ya que no debe usar Xolair.
Advertencias y precauciones
Consulte a su médico antes de usar Xolair:
- si tiene problemas de riñón o hígado,
- si padece una alteración en la que su propio sistema inmune ataca partes de su propio organismo (enfermedad autoinmune),
- si va a viajar a una región donde las infecciones causadas por parásitos son comunes ya que Xolair puede disminuir su resistencia a dichas infecciones,
- si ha tenido una reacción alérgica grave (anafilaxia) previa, por ejemplo como consecuencia del uso de una medicina, una picadura de un insecto o por comida,
- si ha presentado alguna reacción alérgica al látex. El capuchón de la aguja de la jeringa puede contener caucho seco (látex).
Xolair no trata los síntomas del asma agudo, como puede ser un ataque de asma repentino. Por lo tanto Xolair no debe utilizarse para tratar esta clase de síntomas.
Xolair no está destinado para prevenir o tratar otras afecciones de tipo alérgico, como son reacciones alérgicas repentinas, síndrome de hiperinmunoglobulina E (trastorno inmune hereditario), aspergilosis (enfermedad del pulmón causada por un hongo), alergia alimentaria, eczema o fiebre del heno ya que Xolair no se ha estudiado en estas afecciones.
Vigile los signos de reacciones alérgicas y otros efectos adversos graves
Xolair puede ocasionar efectos adversos graves. Usted debe vigilar la aparición de signos de estos efectos mientras use Xolair. Busque asistencia médica de forma inmediata si nota algún signo que indique una reacción alérgica grave u otros efectos adversos graves. Esos signos se mencionan en “Efectos adversos graves” en la sección 4.
Antes de que usted se inyecte Xolair o de que lo haga otra persona distinta a un profesional sanitario, es importante que reciban formación de su médico sobre cómo reconocer los síntomas tempranos de reacciones alérgicas graves y de cómo actuar si se producen (ver sección 3,“Cómo usar Xolair”). La mayoría de las reacciones alérgicas graves ocurren durante las tres primeras dosis de Xolair.
Niños y adolescentes
Asma alérgica
Xolair no está recomendado para niños menores de 6 años de edad. Su uso en niños menores de 6 años de edad no se ha estudiado.
Rinosinusitis crónica con pólipos nasales
Xolair no está recomendado para niños y adolescentes menores de 18 años de edad. Su uso en pacientes menores de 18 años de edad no se ha estudiado.
Urticaria crónica espontánea (UCE)
Xolair no está recomendado para niños menores de 12 años de edad. No se ha estudiado su uso en niños menores de 12 años de edad.
Otros medicamentos y Xolair
Informe a su médico, farmacéutico o enfermero si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Esto es especialmente importante si está utilizando:
- medicamentos para tratar una infección causada por un parásito, ya que Xolair puede reducir el efecto de sus medicamentos,
- corticosteroides inhalados y otros medicamentos para el asma alérgica.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Su médico comentará con usted los beneficios y riesgos potenciales del uso de este medicamento durante el embarazo.
Informe a su médico inmediatamente si queda embarazada mientras está siendo tratada con Xolair.
Xolair puede pasar a la leche materna. Si usted está dando el pecho o tiene intención de hacerlo, consulte a su médico antes de usar este medicamento.
Conducción y uso de máquinas
No es probable que Xolair afecte a su capacidad para conducir y usar máquinas.
3. Cómo usar Xolair
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Cómo se usa Xolair
Xolair se usa como una inyección bajo la piel (conocida como inyección subcutánea).
Inyección de Xolair
- Usted y su médico decidirán si se va a inyectar usted mismo Xolair. Las tres primeras dosis se inyectarán siempre bajo la supervisión de un profesional sanitario (ver sección 2).
- Es importante haber recibido la formación adecuada sobre cómo inyectarse el medicamento antes de que lo haga usted mismo.
- El cuidador (por ejemplo los padres) puede ponerle la inyección de Xolair después de haber recibido la formación apropiada.
Para obtener instrucciones detalladas sobre como inyectar Xolair, ver “Instrucciones de uso de Xolair en jeringa precargada” al final de este prospecto.
Formación para reconocer reacciones alérgicas graves
Es también importante que no se inyecte Xolair a usted mismo hasta que su médico o enfermera no le hayan enseñado:
- cómo reconocer los signos y síntomas tempranos de reacciones alérgicas graves,
- qué hacer si los síntomas aparecen.
Para más información sobre los signos y síntomas tempranos de reacciones alérgicas graves, ver sección 4.
Cuánto se administrará
Asma alérgica y rinosinusitis crónica con pólipos nasales
Su médico decidirá la cantidad de Xolair que necesita y la frecuencia de administración del mismo. Ello depende de su peso corporal y de los resultados de un análisis de sangre realizado antes de iniciar el tratamiento para determinar la concentración de IgE en su sangre.
Necesitará entre 1 y 4 inyecciones al mismo tiempo. Necesitará las inyecciones cada dos o cada cuatro semanas.
Continúe tomando su medicación actual para el asma y/o pólipos nasales mientras dure el tratamiento con Xolair. No interrumpa ninguna medicación para el asma y/o pólipos nasales sin consultarlo con su médico.
Es posible que no perciba una mejoría inmediata después de iniciar el tratamiento con Xolair. En pacientes con pólipos nasales los efectos se han observado 4 semanas después del inicio del tratamiento. En pacientes con asma, por lo general, deben transcurrir entre 12 y 16 semanas hasta que el medicamento surta todo su efecto.
Urticaria crónica espontánea (UCE)
Necesitará dos inyecciones de 150 mg al mismo tiempo cada cuatro semanas.
Continúe tomando su medicación actual para la UCE durante el tratamiento con Xolair. No interrumpa ningún medicamento sin consultarlo con su médico.
Uso en niños y adolescentes
Asma alérgica
Xolair se puede usar en niños y adolescentes a partir de 6 años que ya estén recibiendo medicación para el asma, pero cuyos síntomas asmáticos no están bien controlados por medicamentos como dosis elevadas de esteroides inhalados y beta-agonistas inhalados. Su médico le informará qué cantidad de Xolair necesita su hijo y con qué frecuencia se le debe administrar. Esto dependerá del peso del niño y de los resultados obtenidos de los análisis de sangre realizados antes de iniciar el tratamiento para determinar la cantidad de IgE en su sangre.
No se espera que los niños (de 6 a 11 años de edad) se administren Xolair a ellos mismos. Sin embargo, si el médico lo considera conveniente, el cuidador puede administrarles la inyección después de la formación adecuada.
Rinosinusitis crónica con pólipos nasales
Xolair no se debe usar en niños y adolescentes menores de 18 años de edad.
Urticaria crónica espontánea (UCE)
Xolair se puede usar en adolescentes a partir de 12 años que ya estén recibiendo antihistamínicos pero cuyos síntomas de la UCE no estén bien controlados por estos medicamentos. La dosis para adolescentes a partir de 12 años es la misma que para adultos.
Si olvidó una dosis de Xolair
Si ha olvidado una visita, contacte con su médico u hospital tan pronto como sea posible para volver a programarla.
Si ha olvidado autoinyectarse una dosis de Xolair, inyéctesela tan pronto como lo recuerde. Después consulte con su médico para saber cuándo se deberá administrar la siguiente dosis.
Si interrumpe el tratamiento con Xolair
No interrumpa el tratamiento con Xolair a no ser que se lo indique su médico. La interrupción o finalización del tratamiento con Xolair puede causar una recidiva de sus síntomas.
Sin embargo, si está siendo tratado para la UCE, su médico puede interrumpir el tratamiento con Xolair de vez en cuando para valorar sus síntomas. Siga las instrucciones de su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Los efectos adversos causados por Xolair son, por lo general, de leves a moderados pero ocasionalmente pueden ser graves.
Efectos adversos graves:
Busque atención médica inmediata si usted nota alguno de los signos de los siguientes efectos adversos:
Raros (pueden afectar hasta 1 de cada 1 000 pacientes)
- Reacciones alérgicas graves (incluida anafilaxia). Los síntomas pueden incluir erupción, picor, habones en la piel, hinchazón de la cara, labios, lengua, laringe (caja de voz), tráquea u otras partes del cuerpo, ritmo cardíaco rápido, mareo y ligera sensación de vahído, confusión, disnea, respiración jadeante o dificultad respiratoria, piel o labios azulados, colapso y pérdida de consciencia. Si tiene antecedentes de reacciones alérgicas graves (anafilaxia) no relacionados con Xolair, puede sufrir mayor riesgo de desarrollar una reacción alérgica grave después del uso de Xolair.
- Lupus eritematoso sistémico (LES). Los síntomas pueden incluir dolor muscular, dolor e hinchazón de las articulaciones, erupción, fiebre, pérdida de peso y fatiga.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- Síndrome de Churg-Strauss o síndrome hipereosinofílico. Los síntomas pueden incluir uno o más de los siguientes: hinchazón, dolor o erupción alrededor de los vasos sanguíneos o linfáticos, nivel elevado de un tipo específico de glóbulos blancos (eosinofilia marcada), empeoramiento de los problemas respiratorios, congestión nasal, problemas cardíacos, dolor, adormecimiento, hormigueo en los brazos y piernas.
- Recuento de plaquetas sanguíneas bajo con síntomas como sangrado o hematomas que se producen más fácilmente de lo normal.
- Enfermedad del suero. Los síntomas pueden incluir uno o más de los siguientes: dolor en las articulaciones con o sin inflamación o rigidez, erupción, fiebre, inflamación de los nódulos linfáticos, dolor muscular.
Otros efectos adversos incluyen:
Muy frecuentes (pueden afectar a más de 1 de cada 10 pacientes)
- fiebre (en niños)
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes)
- reacciones en la zona de inyección que incluyen dolor, hinchazón, picor y enrojecimiento
- dolor en la parte superior del estómago
- dolor de cabeza (muy frecuente en niños)
- infección de las vías altas del tracto respiratorio, como es inflamación de la faringe y resfriado común
- sensación de presión o dolor en las mejillas y la frente (sinusitis, dolor de cabeza sinusal)
- dolor en las articulaciones (artralgia)
- sensación de mareo
Poco frecuentes (pueden afectar hasta 1 de cada 100 pacientes)
- sensación de sueño o cansancio
- hormigueo o entumecimiento de manos o pies
- desmayo, disminución de la tensión arterial al sentarse o ponerse de pie (hipotensión postural), rubefacción
- dolor de garganta, tos, problemas respiratorios agudos
- sensación de mareo (nausea), diarrea, indigestión
- picor, habones, erupción, mayor sensibilidad de la piel al sol
- aumento de peso
- síntomas de tipo gripal
- brazos hinchados
Raros (pueden afectar hasta 1 de cada 1 000 pacientes)
- infección parasitaria
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- dolor muscular e inflamación de las articulaciones
- pérdida de pelo
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Xolair
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el etiquetado después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica. El envase que contiene la jeringa precargada puede conservarse durante un total de 48 horas a temperatura ambiente (25 ?C) antes de usar.
- Conservar en el embalaje original para protegerlo de la luz.
- Conservar en nevera (entre 2 °C y 8 °C). No congelar.
- No utilizar ningún envase que esté dañado o muestre indicios de deterioro.
6. Contenido del envase e información adicional
Composición de Xolair
- El principio activo es omalizumab. Una jeringa de 1 ml de solución contiene 150 mg de omalizumab.
- Los demás componentes son hidrocloruro de arginina, hidrocloruro de histidina monohidrato, histidina, polisorbato 20 y agua para preparaciones inyectables.
- La cápsula protectora de la aguja de la jeringa puede contener caucho seco (látex).
Aspecto del producto y contenido del envase
Xolair solución inyectable se presenta como una solución de transparente a ligeramente opalescente, incolora a color amarillo parduzco claro en una jeringa precargada.
Xolair 150 mg solución inyectable en jeringa precargada con aguja fijada de calibre 26 y protector de la jeringa morado está disponible en envases que contienen 1 jeringa precargada y en envases múltiples que contienen 4 (4 x 1), 6 (6 x 1) o 10 (10 x 1) jeringas precargadas.
Puede que no estén comercializados todos los tamaños de envases.
Titular de la autorización de comercialización
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublín 4
Irlanda
Responsable de la fabricación
Novartis Farmacéutica S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
España
Novartis Pharma GmbH
Roonstrasse 25
D-90429 Nuremberg
Alemania
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
Novartis Bulgaria EOOD ???.: +359 2 489 98 28 | Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλ?δα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κ?προς Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
INSTRUCCIONES DE USO DE XOLAIR JERINGA PRECARGADA
Lea TODAS las instrucciones antes de inyectar el medicamento. Si su médico decide que usted o la persona que le cuida puede administrarle sus inyecciones de Xolair en su domicilio, necesitará recibir formación de su médico, enfermera o farmacéutico antes de que usted se inyecte el medicamento o lo inyecte a otros. No se espera que los niños (de 6 años hasta menos de 12 años de edad) se autoinyecten Xolair, sin embargo, si su médico lo considera apropiado, su cuidador puede inyectarles Xolair después de haber recibido la formación adecuada. La caja contiene la(s) jeringa(s) precargada(s) de Xolair cerradas individualmente en una bandeja de plástico.
Su jeringa precargada de Xolair 150mgsolución inyectable




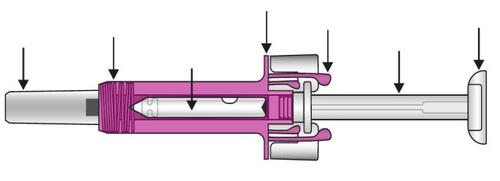
Una vez que el medicamento se haya inyectado, el protector de la jeringa se activará para cubrir la aguja. Éste está concebido para proteger de lesiones causadas por pinchazos accidentales con la aguja.
Qué más necesita para la inyección:
|
|
Información importante de seguridad
Advertencia: Mantenga la jeringa fuera de la vista y del alcance de los niños.
- El capuchón de la aguja de la jeringa puede contener goma seca (látex) que no se debe tocar por las personas sensibles a esta sustancia.
- No abra la caja precintada hasta que esté listo para usar este medicamento.
- No use este medicamento si el precinto de la caja o de la bandeja de plástico están rotos, ya que puede que no sea seguro utilizarlo.
- No utilizar si la jeringa se ha caído sobre una superficie dura o se ha dejado caer después de quitar el capuchón de la aguja.
- Nunca deje la jeringa en lugares donde otras personas puedan tocarla.
- No agite la jeringa.
- Tenga mucho cuidado de no tocar los clips de activación antes de su utilización. Si lo hace, se disparará el protector de la aguja demasiado pronto.
- No quite el capuchón de la aguja hasta justo antes de ponerse la inyección.
- La jeringa no se puede reutilizar. Una vez utilizada, deseche la jeringa al cubo de eliminación de objetos punzantes.
Conservación de Xolair solución inyectable en jeringa precargada
- Conservar este medicamento precintado dentro de su caja para protegerlo de la luz. Conservar en nevera entre 2 °C y 8 °C. NO CONGELAR.
- Recuerde sacar la jeringa de la nevera para que alcance la temperatura ambiente (25ºC) antes de preparar la inyección (esto llevará 30 minutos, aproximadamente). Deje la jeringa en la caja para protegerla de la luz. El tiempo total que la jeringa puede permanecer a temperatura ambiente (25ºC) antes de su uso no debe exceder de 48 horas.
- No utilice la jeringa después de la fecha de caducidad que aparece en la caja o en la etiqueta de la jeringa. Si ha caducado, devuelva el envase completo a la farmacia.
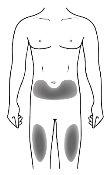
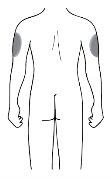
Lugar de inyección
| El lugar de la inyección es el sitio donde usted usará la jeringa
Si quien pone la inyección es el cuidador, entonces también se puede utilizar la parte superior de los brazos. |
Preparación de Xolair solución inyectable en jeringa precargada lista para utilizar
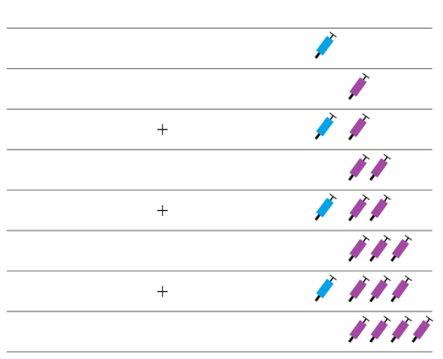
Nota: Dependiendo de la dosis que le haya prescrito su médico, usted puede necesitar preparar una o más jeringas precargadas e inyectar el contenido de todas ellas. La siguiente tabla proporciona ejemplos del número de inyecciones de cada concentración que puede necesitar para una dosis determinada:
|
- Saque de la nevera la caja con la jeringa y déjela sin abrir durante 30 minutos, aproximadamente, hasta que alcance temperatura ambiente (deje la jeringa dentro de la caja para protegerla de la luz).
- Cuando esté listo para utilizar la jeringa, lávese bien las manos con agua y jabón.
- Desinfecte bien la zona de inyección con una toallita humedecida con alcohol.
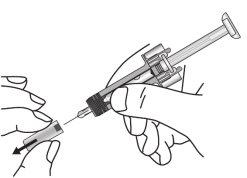
- Saque la bandeja de plástico de la caja y retire el papel que la cubre. Agarre la jeringa por la mitad de su protector azul y extraiga la jeringa de la bandeja.
- Inspeccione la jeringa. El líquido debe ser de transparente a ligeramente turbio. Su color puede variar de incoloro a amarillo parduzco pálido. Puede haber alguna burbuja de aire pequeña, que es normal. NO UTILIZAR si la jeringa está rota o si el líquido está claramente turbio, tiene un color claramente marrón o si contiene partículas. En todos estos casos, devuelva el envase completo a la farmacia.
- Sujete la jeringa horizontalmente para revisar la fecha de caducidad impresa en la etiqueta a través del visor. Nota: es posible rotar la parte interna de la jeringa de modo que la etiqueta se puede leer a través de la ventana del visor. NO UTILIZAR si el medicamento ha caducado. Si ha caducado devuelva el envase completo a la farmacia.
Cómo utilizar la jeringa precargada de Xolair solución inyectable
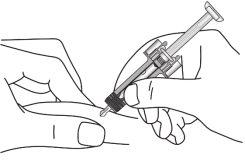
| Retire con cuidado el capuchón de la aguja de la jeringa. Elimine el capuchón. Puede que observe una gota en la punta de la aguja. Esto es normal. |
| Pellizque suavemente la piel del lugar de la inyección e inserte la aguja como muestra la figura. Introduzca la aguja por completo para garantizar que se administra todo el medicamento. |
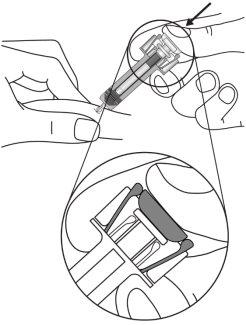
| Sujete la jeringa como se muestra. Presione lentamenteel émbolo hasta el finalde tal manera que la cabeza del émbolo quede encajada en los clips de activación del protector. |
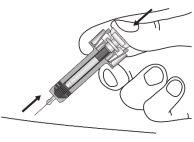
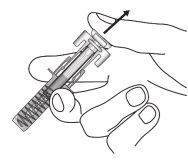
| Mantenga completamente presionado el émbolomientras con cuidado retira la aguja de la zona de inyección. |
| Suelte el émbolo lentamente y deje que el protector de la jeringa tape automáticamente la aguja. Puede que haya un poquito de sangre en la zona de inyección. Puede presionar la zona de inyección con un algodón o una gasa durante 30 segundos. No se frote la zona de inyección. Se puede poner una tirita si lo necesita. |
Instrucciones de eliminación
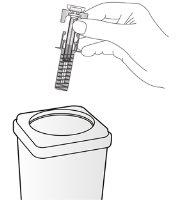
| Deseche la jeringa usada en un contenedor para eliminar objetos punzantes (recipiente cerrado y resistente a pinchazos). Por motivos de seguridad y de salud (de usted y de otras personas), las agujas y las jeringas usadas nunca se deben reutilizar. La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local. Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a XOLAIR 150 MG SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 150 mgPrincipio activo: OmalizumabFabricante: Celltrion Healthcare Hungary Kft.Requiere recetaForma farmacéutica: INYECTABLE, 75 MGPrincipio activo: OmalizumabFabricante: Celltrion Healthcare Hungary Kft.Requiere recetaForma farmacéutica: INYECTABLE, 150 mgPrincipio activo: OmalizumabFabricante: Novartis Europharm LimitedRequiere receta
Médicos online para XOLAIR 150 MG SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de XOLAIR 150 MG SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes