TIVICAY 5 MG COMPRIMIDOS DISPERSABLES

Cómo usar TIVICAY 5 MG COMPRIMIDOS DISPERSABLES
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Tivicay 5 mg comprimidos dispersables
dolutegravir
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento (o su hijo, si es el paciente), porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted (o su hijo, si es el paciente), y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Tivicay y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Tivicay
- Cómo tomar Tivicay
- Posibles efectos adversos
- Conservación de Tivicay
- Contenido del envase e información adicional
También se proporcionan instrucciones de uso paso a paso.
1. Qué es Tivicay y para qué se utiliza
Tivicay contiene como principio activo dolutegravir. Dolutegravir pertenece a un grupo de medicamentos antirretrovirales llamados inhibidores de la integrasa (INIs).
Tivicay se usa para tratar la infección por el VIH(virus de la inmunodeficiencia humana) en adultos, adolescentes y niños de al menos 4 semanas y mayores, que pesen al menos 3 kg.
Tivicay no cura la infección por el VIH; reduce la cantidad de virus en su cuerpo y lo mantiene en un nivel bajo. Como resultado, también aumenta el número de células CD4 en la sangre. Las células CD4 son un tipo de glóbulos blancos que son importantes para ayudar a su cuerpo a combatir las infecciones.
No todas las personas responden al tratamiento con Tivicay de la misma manera. Su médico vigilará la efectividad de su tratamiento.
Tivicay siempre se utiliza en combinación con otros medicamentos antirretrovirales (tratamiento combinado). Para controlar su infección por el VIH y para evitar que su enfermedad empeore, debe seguir tomando todos sus medicamentos, a menos que su médico le haya indicado que deje de tomar alguno de ellos.
2. Qué necesita saber antes de empezar a tomar Tivicay
No tome Tivicay:
- si usted (o su hijo, si es el paciente) es alérgico a dolutegravir o a alguno de los demás componentes de
- este medicamento (incluidos en la sección 6).
- si usted (o su hijo, si es el paciente) está tomando otro medicamento llamado fampridina (también conocido como dalfampridina; utilizado para tratar la esclerosis múltiple).
- Si cree que algo de esto le aplica (o a su hijo), consulte a su médico.
Advertencias y precauciones
Esté atento a los síntomas importantes
Algunas personas que toman medicamentos para la infección por el VIH desarrollan otros trastornos, que pueden ser graves. Estos incluyen:
- síntomas de infecciones e inflamación
- dolor en las articulaciones, rigidez y problemas de huesos.
Usted necesita saber a qué signos y síntomas importantes debe estar atento mientras esté (o su hijo, si es el paciente) tomando Tivicay.
- Lea la información en la sección 4 de este prospecto.
Niños
No dé este medicamento a niños menores de 4 semanas de edad, que pesen menos de 3 kg o con infección por el VIH resistente a otros medicamentos similares a Tivicay. El uso de Tivicay comprimidos dispersables en niños menores de 4 semanas o que pesen menos 3 kg todavía no ha sido estudiado.
Los niños deben asistir a las citas médicas programadas(consulte "Niños y adolescentes" en la sección 3 para obtener más información).
Otros medicamentos y Tivicay
Informe a su médico si está tomando (o su hijo, si es el paciente), ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
No tome Tivicaycon el siguiente medicamento:
- fampridina (también conocido como dalfampridina), utilizado para tratar la esclerosis múltiple.
Algunos medicamentos pueden afectar al funcionamiento de Tivicay, o aumentar la probabilidad de sufrir efectos adversos. Tivicay también puede afectar al funcionamiento de algunos otros medicamentos.
Informe a su médicosi está tomando (o su hijo) alguno de los siguientes medicamentos:
- metformina, para tratar la diabetes
- medicamentos llamados antiácidos, para tratar la indigestióny el ardordeestómago. Notomeunantiácidodurante las 6 horas antes de tomar Tivicay, o por lo menos 2 horas después de tomarlo (ver también sección 3)
- suplementos o preparados multivitamínicos que contengan calcio, hierro o magnesio. Si toma Tivicay con alimentos, puede tomar suplementos o preparados multivitamínicos que contengan calcio, hierro o magnesio al mismo tiempo que Tivicay. Si no toma Tivicay con alimentos, no tome un suplemento o preparado multivitamínico que contenga calcio, hierro o magnesiodurante las 6 horas previas a la toma de Tivicay, o durante al menos 2 horas después de tomarlo (ver también sección 3)
- etravirina, efavirenz, fosamprenavir/ritonavir, nevirapina o tipranavir/ritonavir, para tratar la infección por el VIH
- rifampicina, para tratar la tuberculosis (TB) y otras infecciones bacterianas
- fenitoína y fenobarbital, para tratar la epilepsia
- oxcarbazepina y carbamazepina, para tratar la epilepsiao el trastorno bipolar
- hierba de San Juan(Hypericum perforatum), un medicamento a base de plantas para tratar la depresión.
- Informe a su médico o farmacéuticosi está tomando (o su hijo) alguno de estos medicamentos. Su médico puede decidir ajustar su dosis o que usted necesite chequeos adicionales.
Embarazo
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada:
- Consulte a su médicosobre los riesgos y beneficios de tomar Tivicay.
Informe a su médico de inmediato si se queda embarazada o tiene intención de quedarse embarazada. Su médico revisará su tratamiento. No interrumpa el tratamiento con Tivicay sin consultar a su médico, ya que esto podría dañarles a usted y a su feto.
Lactancia
No se recomiendaque las mujeres que conviven con el VIH den el pecho porque la infección por VIH puede transmitirse al bebé a través de la leche materna.
Una pequeña cantidad de los componentes de Tivicay pueden pasar a la leche materna.
Si está dando el pecho o piensa en dar el pecho, debe consultar con su médico lo antes posible.
Conducción y uso de máquinas
Tivicay puede hacer que se sienta mareado y tiene otros efectos adversos que reducen su atención.
- No conduzca ni maneje maquinaria, a menos que esté seguro de que no le afecta.
Tivicay contiene menos de 1 mmol de sodio (23 mg) por comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Tivicay
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Adultos
- La dosis recomendada en adultos es de 30 mg (tomados como seis comprimidos dispersables de 5 mg) una vez al día.
- Si está tomando además otros medicamentos, la dosis es de de 30 mg (tomados como seis comprimidos dispersables de 5 mg) dos veces al día.
- Para el VIH resistentea otros medicamentos similares a Tivicay, la dosis recomendada es de de 30 mg (tomados como seis comprimidos dispersables de 5 mg) dos veces al día.
Su médico decidirá cúal es la dosis correcta de Tivicay para usted.
Niños y adolescentes
- La dosis infantilde Tivicay se debe ajustar a medida que crecen o aumentan de peso.
- Por lo tanto, es importante que los niños asistan a las citas médicas programadas.
- Los niños y adolescentes que pesen por lo menos 20 kg pueden tomar la dosis de adultos de 30 mg, una vez al día o 15 mg dos veces al día. Su médico decidirá cómo se debe administrar Tivicay.
- Para niños de al menos 4 semanas y que pesen entre 3 y 20 kg, su médico decidirá la dosis correcta de Tivicay, dependiendo del peso y la edad de su hijo.
- Si traga los comprimidos enteros con agua, los niños no deben tragar más de un comprimido a la vez,para reducir el riesgo de atragantamiento.
- Tivicay no debeutilizarse en niños y adolescentes con infección por el VIH resistentea otros medicamentos similares a Tivicay.
Cómo tomar los comprimidos dispersables
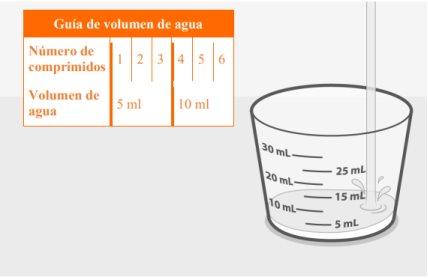
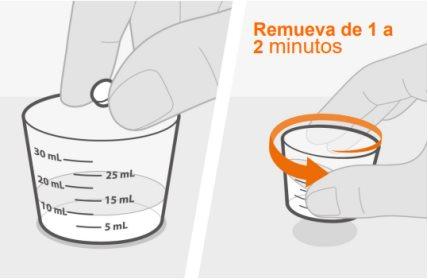
- Los comprimidos dispersables se pueden dispersar en agua o tragarse enteros con agua. Cuando se disperse, la cantidad de agua dependerá de la cantidad de comprimidos prescritos. Los comprimidos deben dispersarse completamente antes de tragarlos.
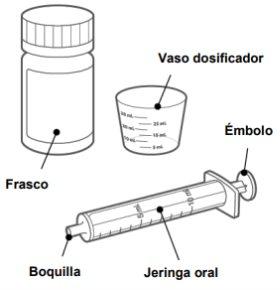
Consulte las instrucciones de usocon respecto a cómo dispersar y administrar los comprimidos utilizando el vaso dosificador y la jeringa oral que se proporcionan en este envase.
- No mastique, corte ni triture los comprimidos.
- Tivicay se puede tomar con o sin alimentos. Cuando tome Tivicay dos veces al día, su médico le puede aconsejar que lo tome con alimentos.
Tivicay también está disponible en comprimidos recubiertos con película. Los comprimidos recubiertos con película y los comprimidos dispersables no son lo mismo, por lo tanto, no cambieentre comprimidos recubiertos con película y comprimidos dispersables sin antes consultar con su médico.
Medicamentos antiácidos
Los antiácidos, utilizados para tratar la indigestióny el ardor de estómago, pueden interrumpir la absorción de Tivicay en su cuerpo y hacer que éste sea menos eficaz.
No tome un antiácidodurante las 6 horas antes de tomar Tivicay, o por lo menos 2 horas después de tomarlo. Otros medicamentos que disminuyen la acidez, como ranitidina y omeprazol, pueden tomarse al mismo tiempo que Tivicay.
- Consulte con su médico para que le aconseje sobre qué medicamentos contra la acidez puede tomar con Tivicay.
Suplementos o preparados multivitamínicosque contengan calcio, hierro o magnesio
Los suplementos o preparados multivitamínicos que contengan calcio, hierro o magnesio pueden interrumpir la absorción de Tivicay en su cuerpo y hacer que éste sea menos eficaz.
Si toma Tivicay con alimentos, puede tomar suplementos o preparados multivitamínicos que contengan calcio, hierro o magnesio al mismo tiempo que Tivicay. Si no toma Tivicay con alimentos, no tome un suplemento o preparado multivitamínico que contenga calcio, hierro o magnesiodurante las 6 horas previas a la toma de Tivicay, o durante al menos 2 horas después de tomarlo.
- Consulte a su médico para que le aconseje sobre cómo tomar suplementos o preparados multivitamínicos que contengan calcio, hierro o magnesio con Tivicay.
Si toma más Tivicay del que debe
Si usted (o su hijo) excede el número de comprimidos de Tivicay, contacte con su médico o farmacéutico para que le asesore. Si es posible, muéstreles la caja de Tivicay.
Si olvidó tomar Tivicay
Si usted (o su hijo) olvida una dosis, tómela tan pronto como lo recuerde. Pero si quedan menos de 4 horas para su próxima dósis, sáltese esa dosis que olvidó y tome la siguiente a la hora habitual. Luego continúe su tratamiento como antes.
- No tome una dosis doblepara compensar las dosis olvidadas.
No interrumpa el tratamiento con Tivicay
Tome Tivicay hasta que su médico se lo indique. No deje de tomarlo a menos que su médico se lo aconseje.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas
Estas son poco frecuentes en personas que toman Tivicay. Los signos incluyen:
- erupción cutánea
- una alta temperatura (fiebre)
- falta de energía (fatiga)
- hinchazón, a veces de la cara o la boca (angioedema), causando dificultad para respirar
- dolores musculares o articulares.
- Acuda a un médico inmediatamente.Su médico puede decidir realizar pruebas hepáticas, de riñón o sanguíneas y puede que le indique que deje de tomar Tivicay.
Efectos adversos muy frecuentes
Estos pueden afectar a más de 1 de cada 10 personas:
- dolor de cabeza
- diarrea
- náuseas.
Efectos adversos frecuentes
Estos pueden afectar hasta 1 de cada 10 personas:
- erupción cutánea
- picor (prurito)
- vómitos
- dolor de estómago (dolor abdominal)
- molestias en el estómago (abdomen)
- aumento de peso
- insomnio
- mareo
- sueños anormales
- depresión (sensación de tristeza profunda y falta de autoestima)
- ansiedad
- falta de energía (fatiga)
- gases (flatulencia)
- aumento en el nivel de las enzimas del hígado
- aumento en el nivel de las enzimas producidas en los músculos (creatinfosfoquinasa).
Efectos adversos poco frecuentes
Estos pueden afectar hasta 1 de cada 100 personas:
- inflamación del hígado (hepatitis)
- intento de suicidio*
- pensamientos suicidas*
- crisis de angustia
- dolor articular
- dolor muscular.
*especialmente en pacientes que anteriormente han tenido depresión o problemas de salud mental.
Efectos adversos raros
Estos pueden afectar hasta 1 de cada 1000 personas:
- insuficiencia del hígado (los signos pueden incluir coloración amarillenta de la piel y del blanco de los ojos u orina inusualmente oscura)
- aumento de bilirrubina (prueba de función del hígado) en su sangre
- suicidio (especialmente en pacientes que anteriormente han tenido depresión o problemas de salud mental).
- Informe a su médico de inmediatosi experimenta algún problema de salud mental (consulte también otros problemas de salud mental que aparecen más arriba).
Síntomas de infección e inflamación
Las personas con infección por el VIH avanzada (SIDA) tienen un sistema inmunitario debilitado y es más probable que desarrollen infecciones graves (infecciones oportunistas). Tales infecciones pueden haberse desarrollado de manera "silenciosa", no siendo detectadas por el sistema inmunitario debilitado antes de que el tratamiento se iniciara. Después de iniciar el tratamiento, el sistema inmunitario se vuelve más fuerte y puede luchar contra estas infecciones, lo que puede causar síntomas de infección o inflamación. Los síntomas generalmente incluyen fiebre, además de algunos de los siguientes:
- dolor de cabeza
- dolor de estómago
- dificultad para respirar.
En casos raros, como el sistema inmunitario se vuelve más fuerte, también puede atacar tejidos sanos (trastornos autoinmunitarios). Los síntomas de los trastornos autoinmunitarios pueden aparecer muchos meses después de comenzar a tomar medicamentos para tratar la infección por el VIH. Los síntomas pueden incluir:
- palpitaciones (latidos cardíacos irregulares o rápidos) o temblor
- hiperactividad (excesiva inquietud y movimiento)
- debilidad que empieza en las manos y pies y que asciende hacia el tronco del cuerpo.
Si usted (o su hijo) tiene cualquier síntoma de infección e inflamacióno si nota cualquiera de los síntomas anteriores:
- Consulte a su médico inmediatamente. No tome otros medicamentos para la infección sin
consultar antes con su médico.
Dolor en las articulaciones, rigidez y problemas de huesos
Algunas personas en tratamiento combinado para el VIH desarrollan osteonecrosis. En esta afección, partes del tejido óseo mueren debido al menor aporte de sangre a los huesos. Las personas pueden ser más propensas a padecer esta afección:
- si han tomado un tratamiento combinado durante un largo tiempo
- si también están tomando medicamentos antiinflamatorios llamados corticosteroides
- si beben alcohol
- si su sistema inmunitario está muy debilitado
- si tienen sobrepeso.
Los signos de la osteonecrosis incluyen:
- rigidez en las articulaciones
- molestias y dolores en las articulaciones (especialmente en la cadera, rodilla u hombro)
- dificultad de movimiento.
Si nota alguno de estos síntomas:
- Informe a su médico.
Efectos en el peso, los lípidos y la glucosa en sangre
Durante el tratamiento frente al VIH puede haber un aumento en el peso y en los niveles de lípidos y glucosa en sangre. Esto está parcialmente relacionado con la recuperación de la salud y el estilo de vida y, a veces, con los propios medicamentos para tratar el VIH. Su médico evaluará estos cambios.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tivicay
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y frasco después de CAD.
Conservar en el embalaje original para protegerlo de la humedad. Mantener el frasco perfectamente cerrado. No tire el desecante. No ingiera el desecante. Este medicamento no requiere ninguna temperatura especial de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Tivicay
- El principio activo es dolutegravir. Cada comprimido contiene dolutegravir sódico equivalente a 5 mg de dolutegravir.
- Los demás componentes son manitol (E421), celulosa microcristalina, povidona, carboximetilalmidón sódico, dióxido de silicio coloidal y celulosa microcristalina, crospovidona, estearil fumarato de sodio, sulfato de calcio dihidrato, sucralosa, aroma a crema de fresa, dióxido de titanio (E171), hipromelosa y macrogol.
Aspecto del producto y contenido del envase
Los comprimidos dispersables de Tivicay 5 mg son blancos, redondos, biconvexos, grabados con “SV H7S” en una cara y “5” en la otra. El frasco contiene un desecante para reducir la humedad. Una vez abierto mantenga el desecante en el frasco, no lo tire.
Los comprimidos dispersables se suministran en frascos que contienen 60 comprimidos.
Con el envase se suministran un vaso dosificador y una jeringa para uso oral.
Titular de la autorización de comercialización
ViiV Healthcare BV
Van Asch van Wijckstraat 55H
3811 LP Amersfoort
Países Bajos
Responsable de la fabricación
Glaxo Wellcome, S.A., Avda. Extremadura 3, 09400 Aranda de Duero (Burgos), España
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien ViiV Healthcare srl/bv Tél/Tel: + 32 (0) 10 85 65 00 | Lietuva ViiV Healthcare BV Tel: + 370 80000334 |
| Luxembourg/Luxemburg ViiV Healthcare srl/bv Belgique/Belgien Tél/Tel: + 32 (0) 10 85 65 00 |
Ceská republika GlaxoSmithKline, s.r.o. Tel: + 420 222 001 111 | Magyarország ViiV Healthcare BV Tel.: + 36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf.: + 45 36 35 91 00 | Malta ViiV Healthcare BV Tel: + 356 80065004 |
Deutschland ViiV Healthcare GmbH Tel.: + 49 (0)89 203 0038-10 | Nederland ViiV Healthcare BV Tel: + 31 (0) 33 2081199 |
Eesti ViiV Healthcare BV Tel: + 372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλáδα GlaxoSmithKline Μονοπρóσωπη A.E.B.E. Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
España Laboratorios ViiV Healthcare, S.L. Tel: + 34 900 923 501 | Polska GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 |
France ViiV Healthcare SAS Tél.: + 33 (0)1 39 17 69 69 | Portugal VIIVHIV HEALTHCARE, UNIPESSOAL, LDA Tel: + 351 21 094 08 01 |
Hrvatska ViiV Healthcare BV Tel: + 385 1 800787089 | România ViiV Healthcare BV Tel: + 40800672524 |
Ireland GlaxoSmithKline (Ireland) Limited Tel: + 353 (0)1 4955000 | Slovenija ViiV Healthcare BV Tel: + 386 80688869 |
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika ViiV Healthcare BV Tel: + 421 800500589 |
Italia ViiV Healthcare S.r.l Tel: + 39 (0)45 7741600 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 (0)10 30 30 30 |
Κúπρος ViiV Healthcare BV Τηλ: + 357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija ViiV Healthcare BV Tel: + 371 80205045 | United Kingdom (Northern Ireland) ViiV Healthcare BV Tel: + 44 (0)800 221441 |
Fecha de la última revisión de este prospecto: {mes AAAA}
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
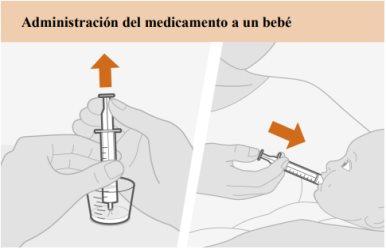
Instrucciones de uso paso a paso
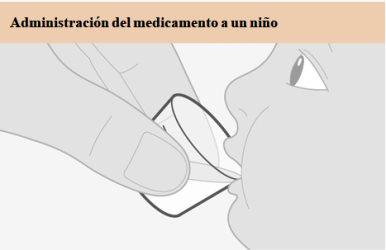
Lea estas instrucciones de uso antes de administrar una dosis de medicamento. Siga los pasos, usando agua potable limpia para preparar y administrar una dosis a un bebé o un niño que no pueda tragar los comprimidos. Información importante Administre siempre este medicamento exactamente como le haya indicado su médico. Hable con él si no está seguro. No mastique, corte ni triture los comprimidos. Si olvidó darle una dosis de medicamento, hágalo tan pronto como lo recuerde. Pero si tiene que tomar su próxima dosis dentro de 4 horas o menos, omita la dosis que olvidó y tome la siguiente a la hora habitual. Luego continúe su tratamiento como antes. No administre 2 dosis al mismo tiempo ni administre más de lo prescrito por su médico. Si administra demasiado medicamento, acuda a urgencias inmediatamente en busca de ayuda médica. Si su hijo puede y prefiere tragar los comprimidos, puede omitir los siguientes pasos.
La guía de volumen de agua anterior muestra la cantidad de agua necesaria para la dosis prescrita. |
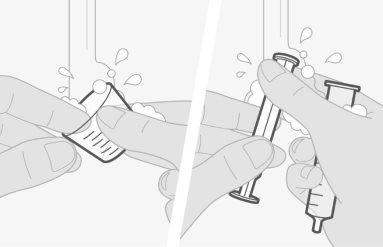
Use solo agua potable. No utiliceninguna otra bebida o alimento para preparar la dosis. |
|
Si derrama algo de medicamento, límpielo. Deseche el resto del medicamento preparado y haga una nueva dosis. |
Debe administrar la dosis del medicamento dentro de los 30 minutos posteriores a la preparación de la dosis.Si han pasado más de 30 minutos, tire la dosis y prepare una nueva dosis de medicamento. |
|
De tiempo a que trague el medicamento. |
Mantener los comprimidos en el frasco. Mantener el frasco perfectamente cerrado. El frasco contiene un desecante que ayuda a mantener secos los comprimidos. Noingiera el desecante. No tireel desecante. Mantener todos los medicamentos fuera del alcance de los niños.
Cuando se hayan acabado todos los comprimidos del frasco o ya no sean necesarios, deseche el frasco, el vasito dosificador y la jeringa oral. Deséchelos siguiendo las directrices locales sobre residuos domésticos. Recibirá un nuevo vaso dosificador y una jeringa oral con su siguiente envase. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TIVICAY 5 MG COMPRIMIDOS DISPERSABLESForma farmacéutica: COMPRIMIDO, 10 mgPrincipio activo: DolutegravirFabricante: Viiv Healthcare B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 25 mgPrincipio activo: DolutegravirFabricante: Viiv Healthcare B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 50 mgPrincipio activo: DolutegravirFabricante: Viiv Healthcare B.V.Requiere receta
Médicos online para TIVICAY 5 MG COMPRIMIDOS DISPERSABLES
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TIVICAY 5 MG COMPRIMIDOS DISPERSABLES, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes