NEULASTA 6 mg INJECTABLE SOLUTION

How to use NEULASTA 6 mg INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Neulasta 6 mg Solution for Injection
pegfilgrastim
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Neulasta and what is it used for
- What you need to know before you use Neulasta
- How to use Neulasta
- Possible side effects
- Storage of Neulasta
- Contents of the pack and other information
1. What is Neulasta and what is it used for
Neulasta contains the active substance pegfilgrastim. Pegfilgrastim is a protein produced by biotechnology in the bacterium E. coli. Pegfilgrastim belongs to a group of proteins called cytokines and is very similar to a natural protein (granulocyte colony-stimulating factor) produced by the body.
Neulasta is used to reduce the duration of neutropenia (low white blood cell count) and the incidence of febrile neutropenia (low white blood cell count and fever) that can occur as a result of cytotoxic chemotherapy (medicines that destroy rapidly dividing cells). White blood cells are important cells that help fight infections. These cells are sensitive to the effects of chemotherapy, which can cause their numbers to decrease. If the number of white blood cells becomes too low, there may not be enough to fight off bacteria, which can lead to a greater risk of infection.
Your doctor has prescribed Neulasta to stimulate your bone marrow (the part of the bone where blood cells are produced) to produce more white blood cells to help fight infections.
2. What you need to know before you use Neulasta
Do not use Neulasta
- if you are allergic to pegfilgrastim, filgrastim, or any of the other ingredients of this medicine
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Neulasta:
- if you experience an allergic reaction that includes weakness, low blood pressure, difficulty breathing, swelling of the face (anaphylaxis), redness, and itching, skin rash, and itching in areas of the skin.
- if you are allergic to latex. The needle cap of the pre-filled syringe contains a latex derivative that may cause severe allergic reactions.
- if you are allergic to acrylic adhesives. The on-body injector uses an acrylic adhesive that may cause allergic reactions.
- if you experience cough, fever, and difficulty breathing. This may be a sign of Acute Respiratory Distress Syndrome (ARDS).
- if you experience any or a combination of the following side effects:
? swelling that may be associated with urinating less frequently, difficulty breathing, swelling, and feeling of abdominal fullness and a general feeling of tiredness.
These may be symptoms of a disease called "Capillary Leak Syndrome" and may cause blood to leak from small blood vessels into other parts of your body. See section 4.
- if you have pain in the upper left abdomen or pain in the tip of the shoulder. This may be a sign of a problem with the spleen (splenomegaly).
- if you have recently had a severe lung infection (pneumonia), fluid in the lungs (pulmonary edema), inflammation of the lungs (interstitial lung disease), or an abnormal chest X-ray result (pulmonary infiltration).
- if you are aware of any changes in your blood cell count (e.g., increased white blood cell count or anemia) or a decrease in your blood platelet count, which can reduce the ability of your blood to clot (thrombocytopenia). Your doctor may want to monitor you more closely.
- if you have sickle cell anemia. Your doctor may monitor your disease more closely.
- if you are a cancer patient with breast or lung cancer, the combined treatment of Neulasta with chemotherapy and/or radiotherapy may increase the risk of developing a pre-cancerous hematological disorder called myelodysplastic syndrome (MDS) or a hematological malignancy called acute myeloid leukemia (AML). Symptoms may include tiredness, fever, easy bruising or bleeding.
- if you have sudden signs of allergy, such as rash, itching, or hives on the skin, swelling of the face, lips, tongue, or other parts of the body, shortness of breath, wheezing, or difficulty breathing, may be signs of a severe allergic reaction.
- if you have symptoms of aortic inflammation (the large blood vessel that carries blood from the heart to the rest of the body), which has been rarely reported in cancer patients and healthy donors. Symptoms may include fever, abdominal pain, general malaise, back pain, and increased inflammatory markers. Inform your doctor if you experience these symptoms.
Your doctor will regularly perform blood and urine tests since Neulasta may damage the small filters inside the kidneys (glomerulonephritis).
With the use of Neulasta, severe skin reactions (Stevens-Johnson syndrome) have been reported. Stop using Neulasta and seek medical attention immediately if you observe any of the symptoms described in section 4.
You should discuss with your doctor the risk of developing blood cancer. If you develop or may develop blood cancer, you should not use Neulasta, unless your doctor advises you to.
Loss of response to pegfilgrastim
If you experience a loss of response or if you do not achieve a response to treatment with pegfilgrastim, your doctor will investigate the causes, including whether you have developed antibodies that may neutralize the activity of pegfilgrastim.
Other medicines and Neulasta
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
Ask your doctor or pharmacist for advice before taking any medicine. Neulasta has not been used in pregnant women. It is important that you inform your doctor if:
- you are pregnant;
- you think you may be pregnant; or
- you are planning to have a baby.
Unless your doctor tells you otherwise, you must stop breastfeeding if you use Neulasta.
Driving and using machines
Neulasta has no or negligible influence on the ability to drive and use machines.
Neulasta contains sorbitol (E420) and sodium
This medicine contains 30 mg of sorbitol in each pre-filled syringe, equivalent to 50 mg/ml. This medicine contains less than 1 mmol of sodium (23 mg) per 6 mg dose; i.e., it is essentially "sodium-free".
3. How to use Neulasta
Neulasta is indicated in patients aged 18 years and older.
Follow the instructions for administration of Neulasta exactly as your doctor has told you. Ask your doctor or pharmacist if you are unsure. The usual dose is a single subcutaneous injection of 6 mg (under the skin), which should be administered at the end of each chemotherapy cycle, at least 24 hours after your last dose of chemotherapy.
Self-injection of Neulasta
Your doctor may consider it more convenient for you to inject Neulasta yourself. Your doctor or nurse will teach you how to do it. Do not attempt to do it unless you have been taught.
For further instructions on how to self-inject Neulasta, read section 6 at the end of this leaflet.
Do not shake Neulasta vigorously as this may affect its activity.
If you use more Neulasta than you should
If you use more Neulasta than you should, tell your doctor, pharmacist, or nurse.
If you forget to use Neulasta
If you are self-injecting and have forgotten to administer your dose of Neulasta, contact your doctor to decide when you should inject the next dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you experience any or a combination of the following side effects:
- swelling that may be associated with urinating less frequently, difficulty breathing, swelling, and feeling of abdominal fullness and a general feeling of tiredness. These symptoms usually develop very quickly.
These may be symptoms of a disease that occurs infrequently (which may affect up to 1 in 100 people) called "Capillary Leak Syndrome" and may cause blood to leak from small blood vessels into other parts of your body and require urgent medical attention.
Very common side effects(may affect more than 1 in 10 people):
- bone pain. Your doctor will inform you about what you can take to relieve the pain.
- nausea and headache.
Common side effects(may affect up to 1 in 10 people):
- with the use of the on-body injector, skin rash, itching, and hives (contact dermatitis/local skin reactions) have been observed.
- injection site pain.
- with the use of the on-body injector, reactions at the application site have been observed, which may include redness, bleeding, bruising, pain, and discomfort.
- general pain and pain in the joints and muscles.
- some changes in your blood may occur, which will be detected by regular blood tests. The number of white blood cells may increase for a short period. The number of platelets may decrease, which can cause bruising.
Uncommon side effects(may affect up to 1 in 100 people):
- allergic reactions, including redness and flushing, rash, and skin inflammation with itching.
- severe allergic reactions, including anaphylaxis (weakness, low blood pressure, difficulty breathing, facial swelling).
- enlargement of the spleen.
- splenic rupture. Some cases of splenic rupture were fatal. It is important that you contact your doctor immediately if you notice pain in the upper left abdomen or left shoulder, as these may be related to a problem with your spleen.
- respiratory problems. If you have a cough, fever, and difficulty breathing, consult your doctor.
- cases of Sweet's syndrome (painful, inflamed, purple-colored lesions on the limbs and sometimes on the face and neck, accompanied by fever) have been reported, but may be related to other factors.
- cutaneous vasculitis (inflammation of the blood vessels in the skin).
- damage to the small filters inside the kidneys (glomerulonephritis).
- redness at the injection site.
- bleeding from the lungs (pulmonary hemorrhage).
- hematological disorders (myelodysplastic syndrome [MDS] or acute myeloid leukemia [AML]).
Rare side effects(may affect up to 1 in 1,000 people):
- aortic inflammation (the large blood vessel that carries blood from the heart to the rest of the body), see section 2.
- bleeding from the lungs (pulmonary hemorrhage).
- Stevens-Johnson syndrome, which may appear as red, circular, or concentric patches, often with central blisters on the trunk, exfoliation, ulcers in the mouth, throat, nose, genitals, and eyes; and may be preceded by fever and flu-like symptoms. Stop using Neulasta if you develop these symptoms and contact your doctor or seek medical attention immediately. See section 2.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Neulasta
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the pre-filled syringe after EXP. The expiry date refers to the last day of the month shown.
Store in a refrigerator (2°C - 8°C).
Neulasta may be stored at room temperature (below 30°C) for a maximum of 3 days. Once a pre-filled syringe has been removed from the refrigerator and reached room temperature (below 30°C), it must be used within 3 days or discarded.
Do not freeze. Neulasta may be used after accidental freezing for a period of less than 24 hours.
Keep the container in the outer carton to protect from light.
Do not use this medicine if you notice that the solution is not entirely clear or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Composition of Neulasta
- The active ingredient is pegfilgrastim. Each prefilled syringe contains 6 mg of pegfilgrastim in 0.6 ml of solution.
- The other components are sodium acetate, sorbitol (E420), polysorbate 20, and water for injectable preparations. See section 2.
Appearance of the Product and Container Contents
Neulasta is a clear, colorless injectable solution in a prefilled syringe (6 mg/0.6 ml).
Each container contains 1 prefilled glass syringe with a stainless steel needle and a needle cap.
The prefilled syringe (packaged with or without a blister) may also be supplied with an automatic needle guard.
Marketing Authorization Holder and Manufacturer
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Marketing Authorization Holder
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Manufacturer
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co Dublin
Ireland
Manufacturer
Amgen NV
Telecomlaan 5-7
1831 Diegem
Belgium
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien s.a. Amgen n.v. Tel/Tél: +32 (0)2 7752711 | Lietuva Amgen Switzerland AG Vilniaus filialas Tel: +370 5 219 7474 |
| Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tel/Tél: +32 (0)2 7752711 |
Ceská republika Amgen s.r.o. Tel: +420 221 773 500 | Magyarország Amgen Kft. Tel.: +36 1 35 44 700 |
Danmark Amgen, filial af Amgen AB, Sverige Tlf: +45 39617500 | Malta Amgen S.r.l. Italy Tel: +39 02 6241121 |
Deutschland Amgen GmbH Tel: +49 89 1490960 | Nederland Amgen B.V. Tel: +31 (0)76 5732500 |
Eesti Amgen Switzerland AG Vilniaus filialas Tel: +372 586 09553 | Norge Amgen AB Tel: +47 23308000 |
| Österreich Amgen GmbH Tel: +43 (0)1 50 217 |
España Amgen S.A. Tel: +34 93 600 18 60 | Polska Amgen Biotechnologia Sp. z o.o. Tel.: +48 22 581 3000 |
France Amgen S.A.S. Tél: +33 (0)9 69 363 363 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 422 0606 |
Hrvatska Amgen d.o.o. Tel: +385 (0)1 562 57 20 | România Amgen România SRL Tel: +4021 527 3000 |
Ireland Amgen Ireland Limited Tel: +353 1 8527400 | Slovenija AMGEN zdravila d.o.o. Tel: +386 (0)1 585 1767 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 |
Italia Amgen S.r.l. Tel: +39 02 6241121 | Suomi/Finland Amgen AB, sivuliike Suomessa/Amgen AB, filial i Finland Puh/Tel: +358 (0)9 54900500 |
Kúπρος C.A. Papaellinas Ltd Τηλ.: +357 22741 741 | Sverige Amgen AB Tel: +46 (0)8 6951100 |
Latvija Amgen Switzerland AG Rigas filiale Tel: +371 257 25888 | United Kingdom (Northern Ireland) Amgen Limited Tel: +44 (0)1223 420305 |
Date of the Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu/.
Instructions for Injecting Neulasta using a Prefilled Syringe
This section contains information on how to self-inject Neulasta. It is important that you do not attempt to self-inject unless you have received specific training on how to do so from your doctor, nurse, or pharmacist. If you have any doubts about how to administer the injection, ask your doctor, nurse, or pharmacist.
How should you or the person injecting you use the Neulasta Prefilled Syringe?
The injection will be given into the tissue under the skin. This is called a subcutaneous injection.
Equipment Needed for Administration
To give a subcutaneous injection, you will need:
- a Neulasta prefilled syringe; and
- alcohol swab or similar.
What should you do before giving a subcutaneous injection of Neulasta?
- Take the prefilled syringe out of the refrigerator.
- Do not shake the prefilled syringe.
- Do notremove the needle cap until you are ready to give the injection.
- Check the expiration date (EXP) on the prefilled syringe label. Do not use it if it has passed the last day of the month shown.
- Check the appearance of Neulasta. It should be a clear and colorless liquid. Do not use it if you see particles.
- To make the injection more comfortable, let the prefilled syringe come to room temperature for 30 minutes or hold it in your closed hand for a few minutes. Do notheat Neulasta in any other way (for example, do not put it in the microwave or in hot water).
- Wash your hands carefully.
- Find a comfortable and well-lit place and put everything you need within reach.
How to prepare the Neulasta injection?
Before injecting Neulasta, you must do the following:
|
|
- There may be a small air bubble in the prefilled syringe. It is not necessary to remove it before injection. Injecting the solution with an air bubble is not harmful.
- The prefilled syringe is now ready to use.
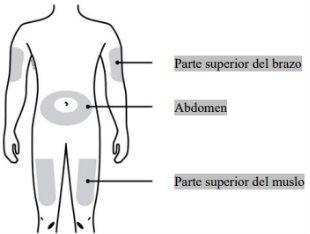
Where should you inject Neulasta?
| The most suitable places to inject yourself are:
If the injection is given by another person, it can also be given in the back of the arms. |
How to give the injection?
- Clean the skin with an alcohol swab.
- Pinch (without squeezing) the skin using your thumb and index finger. Insert the needle into the skin.
- Press the plunger head with a light and constant pressure. Press the plunger head until all the liquid is injected from the syringe.
- After injecting the solution, remove the needle and release the skin.
- If you see a drop of blood at the injection site, remove it with a cotton ball or gauze. Do not rub the injection site. If necessary, you can cover the injection site with a band-aid.
- Do not use the remaining Neulasta left in the syringe.
Remember
Use each syringe for a single injection. If you have any problems, do not hesitate to ask for help and advice from your doctor or nurse.
Disposing of Used Syringes
- Do not put the cap back on used needles.
- Keep used syringes out of sight and reach of children.
- Used syringes must be disposed of according to local regulations. Ask your pharmacist how to dispose of containers and medications that are no longer needed. This will help protect the environment.
Instructions for use | |
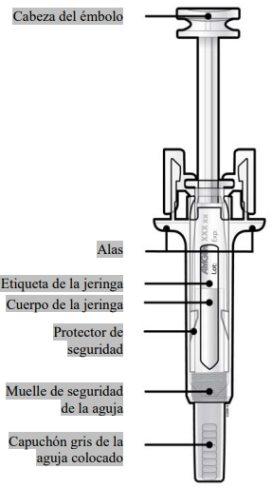
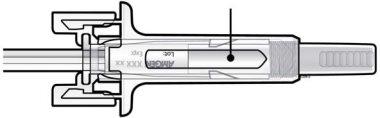
Guide to components | |
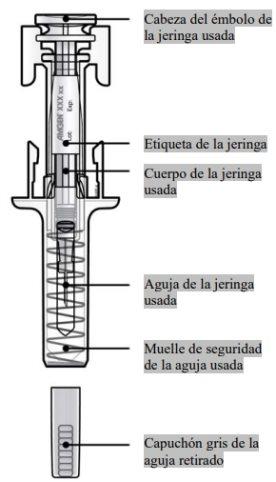
Before use | After use |
|
|
Important |
Read this important information before using the Neulasta prefilled syringe with automatic needle guard:
Do notremove the gray needle cap from the prefilled syringe until you are ready to give the injection. Do notuse the prefilled syringe if it has been dropped onto a hard surface. Use a new prefilled syringe and contact your doctor or healthcare professional. Do notattempt to activate the prefilled syringe before injection. Do notattempt to remove the transparent safety guard from the prefilled syringe. Do notattempt to remove the label from the prefilled syringe body before administering the injection. If you have any doubts, contact your doctor or healthcare professional. |
Step 1: Preparation | |
A | Remove the syringe from the packaging and take the materials you need for your injection: alcohol swabs, cotton balls or gauze, band-aids, and a sharps container (not included). |
To make the injection less painful, let the prefilled syringe come to room temperature for about 30 minutes before the injection. Wash your hands carefully with soap and water. Place the new prefilled syringe and other materials on a clean and well-lit surface. Do notattempt to heat the syringe using a heat source such as hot water or a microwave. Do notleave the prefilled syringe exposed to direct sunlight. Do notshake the prefilled syringe.
|
B | Open the packaging by removing the cover. Take the prefilled syringe by the safety guard to remove it from the tray. |
Hold it here For safety reasons: Do nothold it by the plunger head. Do nothold it by the gray needle cap. |
C | Examine the medication and the prefilled syringe. |
Medication
| |
Do notuse the prefilled syringe if:
In any of these cases, contact your doctor or healthcare professional. |
Step 2: Prepare | |
A | Wash your hands carefully. Prepare and clean the injection site. |
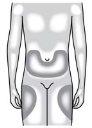
You can inject the medication into: The upper thigh. The abdomen, except for an area of 5 cm (2 inches) around the navel. The outer aspect of the upper arm (only if the injection is given by another person). Clean the injection site with an alcohol swab. Let the skin dry. Do nottouch the injection site before injecting | |
Do notinject into areas where the skin is sensitive, bruised, red, or hardened. Avoid injecting into areas with scars or stretch marks. |
B | Gently pull the gray needle cap straight off in a straight line, keeping the syringe away from your body. |
|
C | Pinch the injection site to create a firm surface. |
| |
|
Step 3: Inject | |
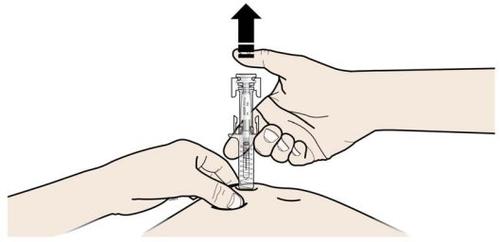
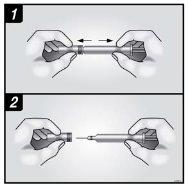
A | Keep the skin pinched. INSERT the needle into the skin. |
Do nottouch the clean area of the skin |
B | PRESS the plunger head with a light and constant pressure until you feel or hear a “click”. Push all the way down until you hear the “click”. |
| |
|
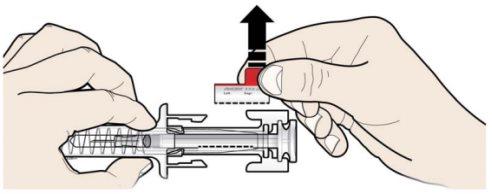
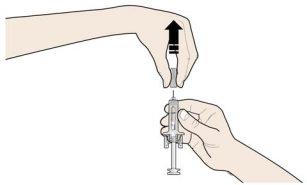
C | RELEASE the plunger head. Then, REMOVE the syringe from the skin. |
After releasing the plunger head, the safety guard of the prefilled syringe will safely cover the needle. Do notput the gray needle cap back on the used prefilled syringe. |
For Healthcare Professionals Only The trade name of the administered product must be correctly recorded in the patient's medical history. |
Remove and store the label from the prefilled syringe.
Turn the plunger to move the label of the syringe to a position where you can remove it. |
Step 4: Finish | |
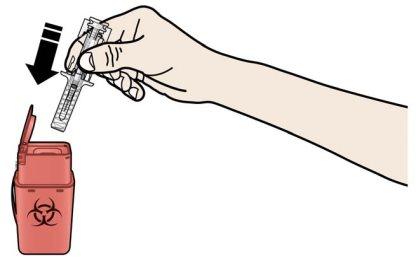
A | Dispose of the used prefilled syringe and other materials in a sharps container. |
Medications must be disposed of according to local regulations. Ask your pharmacist how to dispose of containers and medications that are no longer needed. This will help protect the environment. Keep the syringe and the sharps container out of sight and reach of children. Do notreuse the prefilled syringe. Do notrecycle the prefilled syringes or throw them in the trash. |
B | Examine the injection site. |
If you see blood, press with a cotton ball or gauze on the injection site. Do not rub the injection site. If necessary, put a band-aid. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NEULASTA 6 mg INJECTABLE SOLUTIONDosage form: INJECTABLE, 6 mgActive substance: pegfilgrastimManufacturer: Biosimilar Collaborations Ireland LimitedPrescription requiredDosage form: INJECTABLE, 6 mgActive substance: pegfilgrastimManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 6 mgActive substance: pegfilgrastimManufacturer: Pfizer Europe Ma EeigPrescription required
Online doctors for NEULASTA 6 mg INJECTABLE SOLUTION
Discuss questions about NEULASTA 6 mg INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







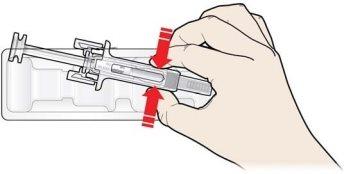

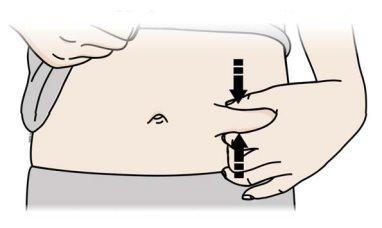
 It is important to keep the skin pinched when injecting.
It is important to keep the skin pinched when injecting.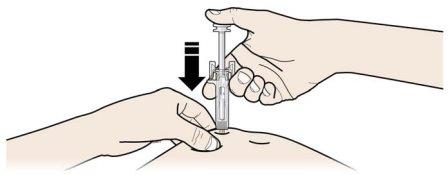
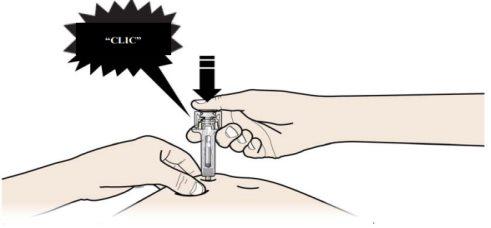
 It is important to press down until you hear the “click” to receive your full dose.
It is important to press down until you hear the “click” to receive your full dose.