NYVEPRIA 6 mg INJECTABLE SOLUTION

How to use NYVEPRIA 6 mg INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Nyvepria 6 mg Solution for Injection
pegfilgrastim
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Nyvepria and what is it used for
- What you need to know before you use Nyvepria
- How to use Nyvepria
- Possible side effects
- Storage of Nyvepria
- Contents of the pack and other information
1. What is Nyvepria and what is it used for
Nyvepria contains the active substance pegfilgrastim. Nyvepria is used in patients treated with cytotoxic chemotherapy (medicines that destroy rapidly dividing cells) to reduce the duration of neutropenia (low count of neutrophils, a type of white blood cell) and to help prevent febrile neutropenia (low count of white blood cells with fever). Nyvepria is indicated in patients aged 18 years and older.
White blood cells are important for fighting infections. If the white blood cell count becomes too low due to cytotoxic chemotherapy, your body may not be able to fight off microorganisms, which can increase the risk of infection. Pegfilgrastim is very similar to a natural protein in the body called granulocyte-colony stimulating factor and works by stimulating your bone marrow to produce more white blood cells to help fight infections.
2. What you need to know before you use Nyvepria
Do not use Nyvepria
- if you are allergic to pegfilgrastim, filgrastim, or any of the other ingredients of this medicine (listed in section 6).
Warnings and Precautions
Consult your doctor, pharmacist, or nurse before using Nyvepria:
- if you have recently had a severe lung infection (pneumonia), fluid in the lungs (pulmonary edema), inflammation of the lungs (interstitial lung disease), or an abnormal chest X-ray result (pulmonary infiltrate).
- if you are aware of any changes in your blood cell count (e.g., increased white blood cell count or anemia) or a decrease in your blood platelet count (thrombocytopenia), which can reduce the ability of your blood to clot. Your doctor may want to monitor you more closely.
- if you have sickle cell anemia. Your doctor may monitor your condition more closely.
Consult your doctor, pharmacist, or nurse while using Nyvepria:
- if you are a cancer patient with breast cancer or lung cancer, the combined treatment of pegfilgrastim with chemotherapy and/or radiotherapy may increase the risk of developing a pre-cancerous blood disorder called myelodysplastic syndrome (MDS) or a blood cancer called acute myeloid leukemia (AML). Symptoms may include fatigue, fever, easy bruising or bleeding.
- if you have an allergic reaction that includes weakness, low blood pressure, difficulty breathing, swelling of the face, lips, tongue, or other parts of the body (anaphylaxis), flushing, and skin rash with itching.
- if you experience cough, fever, and difficulty breathing. This may be a sign of acute respiratory distress syndrome (ARDS).
- if you experience any of the following side effects:
- inflammation or swelling, decreased urination, difficulty breathing, abdominal swelling and feeling of fullness, and general feeling of tiredness.
These may be symptoms of a condition called "capillary leak syndrome" that can cause blood to leak from small blood vessels into other parts of your body. See section 4.
- if you have pain in the upper left abdomen or pain in the left shoulder tip. This may be a sign of a problem with your spleen (splenomegaly).
- if you have fever, abdominal pain, general discomfort, and back pain, as these may be symptoms of inflammation of the aorta (the large blood vessel that carries blood from the heart to the rest of the body). This condition can occur rarely in cancer patients and healthy donors.
Your doctor will perform regular blood and urine tests since Nyvepria may harm your kidneys (glomerulonephritis).
With the use of pegfilgrastim, serious skin reactions (Stevens-Johnson syndrome; a condition that causes painful blisters and ulcers on the skin and mucous membranes, especially in the mouth) have been reported. Stop using Nyvepria and seek medical attention immediately if you notice any of these symptoms: red, often circular, patches on the trunk, exfoliation, ulcers in the mouth, throat, nose, genitals, and eyes, possibly with fever and flu-like symptoms beforehand. See section 4.
You should discuss with your doctor the risk of developing blood cancer. If you have blood cancer or your doctor has told you that you are at risk of developing it, you should not use Nyvepria, unless your doctor advises you to.
Loss of Response to Pegfilgrastim
If treatment with pegfilgrastim does not work or stops working, your doctor will investigate the reason, including whether you have developed antibodies that may neutralize the activity of pegfilgrastim.
Children and Adolescents
Nyvepria is not recommended for use in children and adolescents because there is not enough information on its safety and effectiveness.
Other Medicines and Nyvepria
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Nyvepria has not been studied in pregnant women. Therefore, your doctor may decide that you should not use this medicine.
If you become pregnant during treatment with Nyvepria, inform your doctor.
Unless your doctor tells you otherwise, you must stop breastfeeding if you use Nyvepria.
Driving and Using Machines
Nyvepria has no or negligible influence on the ability to drive and use machines.
Nyvepria Contains Sorbitol (E420) and Sodium
This medicine contains 30 mg of sorbitol in each pre-filled syringe, equivalent to 50 mg/ml. The cumulative effects of administration with other medicines containing sorbitol (or fructose) and dietary intake of sorbitol (or fructose) should be taken into account.
This medicine contains less than 23 mg of sodium (1 mmol) per 6 mg dose; this is essentially "sodium-free".
3. How to Use Nyvepria
Nyvepria is indicated in patients aged 18 years and older.
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is a single subcutaneous injection of 6 mg (under the skin) using a pre-filled syringe, which should be administered at the end of each chemotherapy cycle, at least 24 hours after your last dose of chemotherapy.
Self-Administration of Nyvepria
Your doctor may consider that you can inject Nyvepria yourself. Your doctor or nurse will teach you how to do it. Do not attempt to do it if you have not been taught.
For instructions on how to inject Nyvepria yourself, read the section 6 at the end of this leaflet.
Do not shake Nyvepria vigorously, as this may affect its activity.
If You Use More Nyvepria Than You Should
If you use more Nyvepria than you should, consult your doctor, pharmacist, or nurse.
If You Forget to Use Nyvepria
If you have missed your dose of Nyvepria, contact your doctor to decide when you should have your next injection.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you experience any of the following side effects:
- inflammation or swelling, decreased urination, difficulty breathing, abdominal swelling and feeling of fullness, and general feeling of tiredness. These symptoms usually develop rapidly.
These may be symptoms of a condition called "capillary leak syndrome" that can cause blood to leak from small blood vessels into other parts of your body and may require urgent treatment.
Very Common: may affect more than 1 in 10 people
- bone pain. Your doctor will inform you about what you can take to relieve the pain.
- nausea and headache.
Common: may affect up to 1 in 10 people
- injection site pain.
- general pain and pain in the joints and muscles.
- some changes in your blood may occur, which will be detected by regular blood tests. The number of white blood cells may increase for a short period. The number of platelets may decrease, which can cause bruising.
- chest pain not related to heart problems.
Uncommon: may affect up to 1 in 100 people
- allergic reactions, including flushing, rash, and itching.
- severe allergic reactions, including anaphylaxis (weakness, low blood pressure, difficulty breathing, swelling of the face).
- increase in the size of the spleen (the spleen is an organ located in the abdomen to the left of the stomach that is involved in the production and removal of blood cells and is part of the immune system). Tell your doctor if you experience an increase in size in the upper left part of your abdomen.
- rupture of the spleen, which can be fatal. It is important that you contact your doctor immediately if you have pain in the upper left part of your abdomen or in the left shoulder tip, as these may be related to a problem with your spleen.
- respiratory problems. If you have cough, fever, and difficulty breathing, consult your doctor.
- cases of Sweet's syndrome (painful, inflamed, purple-colored lesions on the limbs and sometimes on the face and neck, accompanied by fever) have been reported.
- cutaneous vasculitis (inflammation of the blood vessels in the skin).
- kidney damage (glomerulonephritis).
- redness at the injection site.
- bleeding from the lungs (hemoptysis).
- hematological disorders (myelodysplastic syndrome [MDS] or acute myeloid leukemia [AML]).
Rare: may affect up to 1 in 1,000 people
- inflammation of the aorta (the blood vessel that carries blood from the heart to the rest of the body), see section 2.
- bleeding from the lungs (pulmonary hemorrhage).
- Stevens-Johnson syndrome, which may appear as red, often circular, patches on the trunk, exfoliation, ulcers in the mouth, throat, nose, genitals, and eyes, possibly with fever and flu-like symptoms beforehand. Stop using Nyvepria and seek medical attention immediately if you develop these symptoms. See section 2.
Reporting of Side Effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Nyvepria
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the pre-filled syringe after EXP. The expiry date refers to the last day of the month shown.
Store in a refrigerator (2°C - 8°C).
Nyvepria may be stored at room temperature (below 25°C) for a maximum of 15 days. Once a pre-filled syringe has been removed from the refrigerator and reaches room temperature (below 25°C), it must be used within 15 days or discarded.
Do not freeze. Nyvepria can be used after accidental freezing for a period of less than 24 hours.
Keep the container in the outer carton to protect from light.
Do not use this medicine if you notice that the solution is not entirely clear or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Composition of Nyvepria
- The active substance is pegfilgrastim. Each pre-filled syringe contains 6 mg of pegfilgrastim in 0.6 ml of solution.
- The other ingredients are sodium acetate trihydrate, glacial acetic acid, sorbitol (E420), polysorbate 20, and water for injectable preparations (see section 2 "Nyvepria contains sorbitol (E420) and sodium acetate").
Appearance and Container Contents of the Product
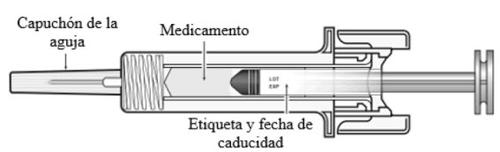
Nyvepria is a clear, colorless, and particle-free injectable solution in a pre-filled syringe (6 mg/0.6 ml).
Each pack contains 1 pre-filled glass syringe with a stainless steel needle, a needle cap, and an automatic needle guard.
Marketing Authorization Holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelles
Belgium
Manufacturer
Hospira Zagreb d.o.o.
Prudnicka cesta 60
10291 Prigorje Brdovecko
Croatia
You can request more information about this medicine by contacting the local representative of the marketing authorization holder.
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tel: + 32 (0)2 554 62 11 | K?προς PFIZER EΛΛAΣ A.E. (CYPRUS BRANCH) T??: + 357 22 817690 |
Ceská Republika Pfizer, spol. s r.o. Tel: + 420-283-004-111 | Magyarország Pfizer Kft. Tel.: + 36 1 488 3700 |
Danmark Pfizer ApS Tlf: + 45 44 20 11 00 | Malta Drugsales Ltd Tel: + 356 21 419 070/1/2 |
Deutschland PFIZER PHARMA GmbH Tel: + 49 (0)30 550055-51000 | Nederland Pfizer bv Tel: + 31 (0)10 406 43 01 |
???????? ??????? ?????????? ????, ???? ???????? Te?: + 359 2 970 4333 | Norge Pfizer AS Tlf: + 47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: + 372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: + 43 (0)1 521 15-0 |
Ελλ?δα PFIZER EΛΛAΣ A.E. Τηλ.: + 30 210 67 85 800 | Polska Pfizer Polska Sp. z o.o. Tel.: + 48 22 335 61 00 |
España Pfizer, S.L. Tel: + 34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: + 351 21 423 5500 |
France Pfizer Tél: + 33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L Tel: + 40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: + 386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Tel: + 1800 633 363 (toll free) Tel: + 44 (0)1304 616161 | Slovenská Republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Ísland Icepharma hf. Tel: + 354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: + 358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: + 39 06 33 18 21 | Sverige Pfizer AB Tel: + 46 (0)8 550 520 00 |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel: + 371 67035775 | United Kingdom (Northen Ireland) Pfizer Limited Tel: + 44 (0)1304 616161 |
Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel: + 3705 2514000 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu.
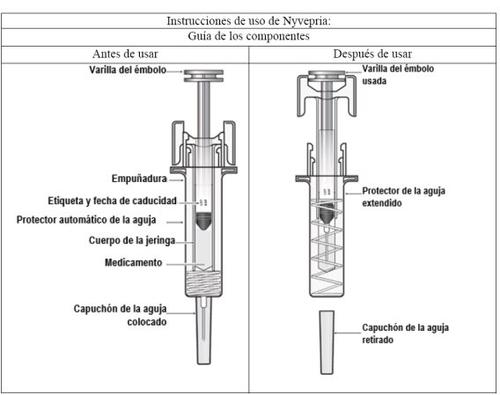
Important |
Read this important information before using the Nyvepria pre-filled syringe with automatic needle guard:
If you have any doubts, contact your doctor or healthcare professional. |
Step 1: Preparation | |
A | Remove the pre-filled syringe from the refrigerator. Remove the inner packaging of the pre-filled syringe from the outer packaging by removing the cover and take the materials you need for your injection: alcohol swabs, cotton balls or gauze, band-aids, and a sharps container (not included). |
To make the injection less painful, let the pre-filled syringe stand at room temperature (not above 25°C) for about 30 minutes before injection. Wash your hands carefully with soap and water. Place the new pre-filled syringe and other materials on a clean and well-lit surface.
|
B | Open the inner packaging of the pre-filled syringe by removing the cover. Hold the pre-filled syringe by the automatic needle guard to remove it from the packaging.
|
For safety reasons:
|
C | Inspect the medicine and the pre-filled syringe. |
| |
In any of these cases, contact your doctor or healthcare professional. |
Step 2: Preparation | |
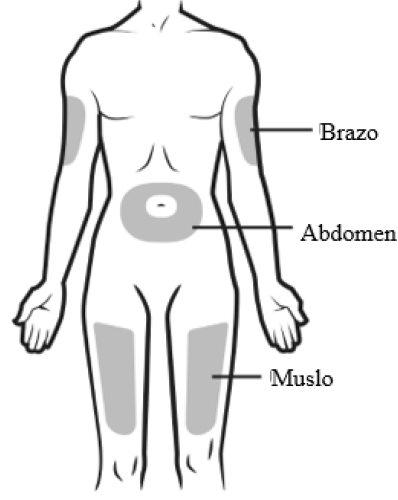
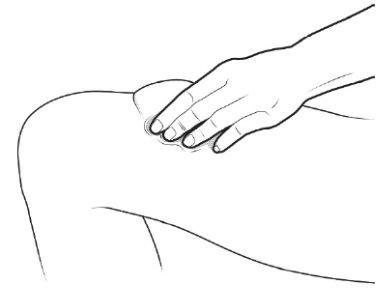
A | Wash your hands carefully. Prepare and clean the injection site. |
You can inject the medicine into:
Clean the injection site with an alcohol swab. Let the skin dry.
Do notinject into areas where the skin is sensitive, bruised, red, or hardened. Avoid injecting into areas with scars or stretch marks. |
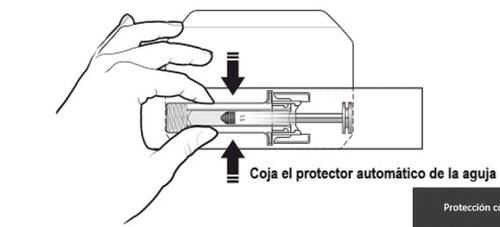
B | Hold the pre-filled syringe by the automatic needle guard. Carefully pull the needle cap straight off the pre-filled syringe, keeping the syringe away from your body. Dispose of the needle cap in the sharps container. Do notreplace the cap. |
|
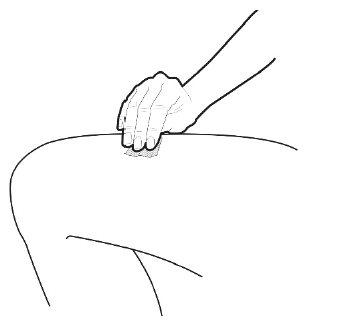
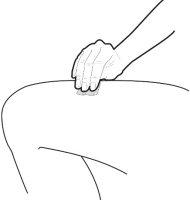
C | Pinch the injection site to create a firm surface. |
It is important to keep the skin pinched when injecting. |
Step 3: Injection | |
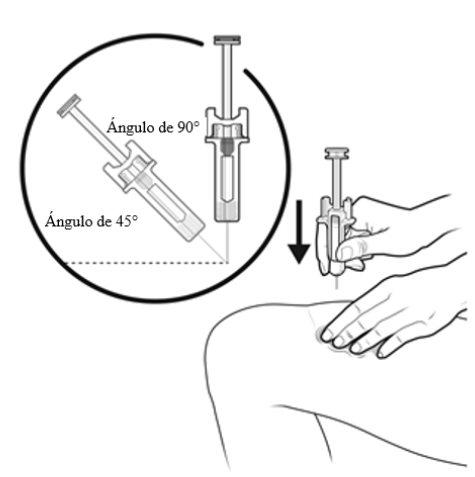
A | Keep the skin pinched. INSERT the needle into the skin at an angle of 45 to 90 degrees. |
|
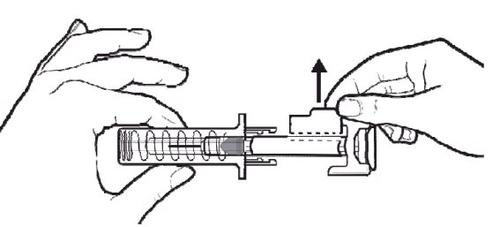
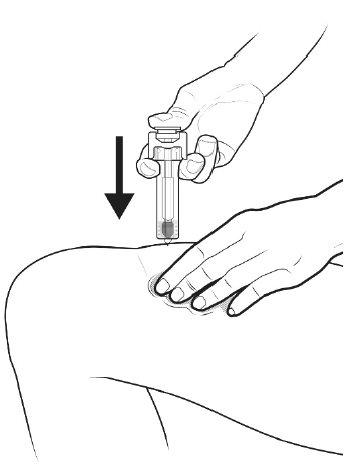
B | PRESS the plunger rod with a light and constant pressure until it reaches the bottom. |
|
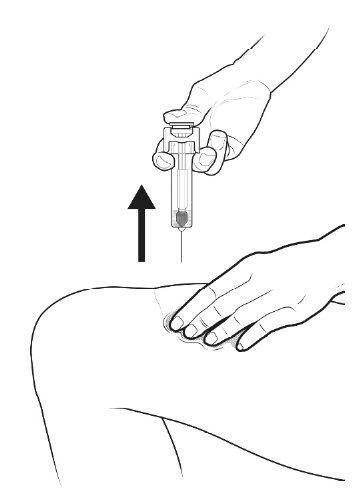
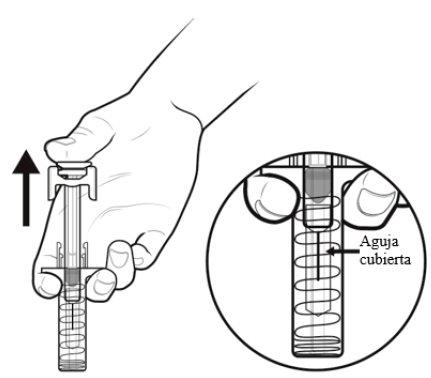
C | When the syringe is empty, REMOVE the syringe from the skin. |
After releasing the plunger rod, the automatic needle guard of the pre-filled syringe will safely cover the needle.
When you remove the syringe, if it appears that the medicine is still in the syringe body, this means you have not received a full dose. Call your doctor or healthcare professional immediately. |
For Healthcare Professionals Only The trade name and batch number of the administered product must be clearly recorded in the patient's medical history. |
Remove and store the pre-filled syringe label.
Turn the plunger rod to move the label of the pre-filled syringe to a position where you can remove it. |
Step 4: Completion | |
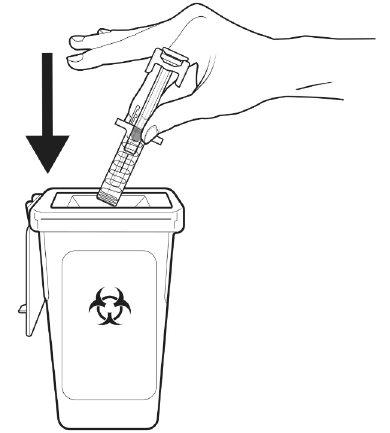
A | Discard the used pre-filled syringe and other materials in a sharps container. |
Medicines should be disposed of in accordance with local regulations. Ask your pharmacist how to dispose of medicines no longer needed. This will help protect the environment. Keep the pre-filled syringe and the sharps container out of the sight and reach of children.
|
B | Check the injection site. |
If you see blood, press a cotton ball or gauze on the injection site. Do notrub the injection site. If necessary, apply a band-aid. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NYVEPRIA 6 mg INJECTABLE SOLUTIONDosage form: INJECTABLE, 6 mgActive substance: pegfilgrastimManufacturer: Biosimilar Collaborations Ireland LimitedPrescription requiredDosage form: INJECTABLE, 6 mg of pegfilgrastim (10mg/ml)Active substance: pegfilgrastimManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 6 mgActive substance: pegfilgrastimManufacturer: Amgen Europe B.V.Prescription required
Online doctors for NYVEPRIA 6 mg INJECTABLE SOLUTION
Discuss questions about NYVEPRIA 6 mg INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






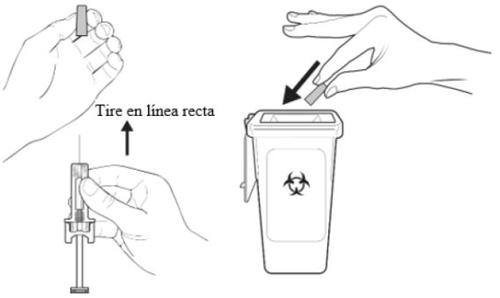

 Do not replace the needle cap on the used pre-filled syringe.
Do not replace the needle cap on the used pre-filled syringe.