
MERIOFERT KIT 150 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use MERIOFERT KIT 150 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Meriofert Kit 150 IUpowder and solvent for solution for injection
menotropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- In this leaflet, Meriofert 150 IU powder and solvent for solution for injection is referred to as Meriofert Kit.
Contents of the pack
- What Meriofert Kit is and what it is used for
- What you need to know before you use Meriofert Kit
- How to use Meriofert Kit
- Possible side effects
- Storing Meriofert Kit
- Contents of the pack and other information
1. What Meriofert Kit is and what it is used for
- Meriofert Kit is used to stimulate ovulation in women who do not ovulate and have not responded to other treatment (clomiphene citrate).
responded to other treatment.
- Meriofert Kit is used to induce the development of multiple follicles (and therefore multiple eggs) in women undergoing fertility treatment.
Meriofert Kit is a highly purified human menopausal gonadotropin, which belongs to a group of medicines called gonadotropins.
Each vial contains lyophilized powder with 150 IU of human follicle-stimulating hormone (FSH) activity and 150 IU of human luteinizing hormone (LH) activity.
The human menopausal gonadotropin (HMG) is extracted from the urine of post-menopausal women. Human chorionic gonadotropin (hCG) is added, which is extracted from the urine of pregnant women, to contribute to the total LH activity.
This medicine should be used under the supervision of a doctor.
2. What you need to know before you use Meriofert Kit
Before starting treatment, your fertility and that of your partner will be assessed.
Do not use Meriofert Kit
- If you have enlarged ovaries or cysts of unknown cause (polycystic ovary syndrome).
- If you have bleeding of unknown origin.
- If you have ovarian, uterine, or breast cancer.
- If you have an abnormal swelling (tumor) of the pituitary gland or hypothalamus (brain).
- If you are allergic to menotropin or any of the other components of this medicine (listed in section 6).
Do not use this medicine if you have premature menopause, malformation of the genital organs, or certain uterine tumors that would prevent a normal pregnancy.
Warnings and precautions
Talk to your doctor before starting Meriofert Kit.
Although allergic reactions to Meriofert Kit have not been described, you should inform your doctor if you have any allergic reactions to similar medicines.
This treatment increases the risk of a disease called ovarian hyperstimulation syndrome (OHSS)(see section 4). If ovarian hyperstimulation occurs, treatment will be stopped and pregnancy should be avoided. The first signs of ovarian hyperstimulation are pain in the lower abdomen, nausea (discomfort), vomiting, and weight gain. If these symptoms occur, you should see a doctor as soon as possible. In severe cases, the ovaries may become enlarged and fluid may accumulate in the abdomen or chest.
The medicine used to trigger the final release of mature eggs (which contains human chorionic gonadotropin, hCG) may increase the likelihood of OHSS. Therefore, it is not recommended to use hCG in cases where OHSS is occurring, and sexual intercourse should not be maintained for at least 4 days, even if a barrier contraceptive method is used.
It is noteworthy that women with fertility problems have a higher rate of miscarriages than the normal population.
The frequency of pregnancies and multiple births in patients undergoing ovulation stimulation treatment is higher than in women who conceive naturally. However, this risk can be minimized if the recommended dose is used.
The risk of ectopic pregnancy (pregnancy outside the uterus) is slightly higher in women with damaged fallopian tubes.
Multiple pregnancies and the characteristics of parents undergoing fertility treatment (e.g., the mother's age or semen characteristics) may be associated with an increased risk of birth defects.
Treatment with Meriofert Kit, like pregnancy itself, can increase the likelihood of suffering a blood clot. A blood clot is the formation of a blood clot in a blood vessel, most often in the veins of the legs or lungs.
Discuss this with your doctor before starting treatment, especially:
- If you already know you have a higher risk of suffering a blood clot.
- If you or a close family member has ever suffered a blood clot.
- If you have severe obesity.
Pediatric population
This medicine is not indicated for pediatric use.
Using Meriofert Kit with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Driving and using machines
Meriofert Kit has no influence or negligible influence on the ability to drive and use machines.
Meriofert Kit contains sodium
This medicine contains less than 23 mg (1 mmol) of sodium per dose, which is essentially "sodium-free".
3. How to use Meriofert Kit
Follow the instructions for administration of this medicine exactly as prescribed by your doctor. If you are unsure, consult your doctor again.
Dose and duration of treatment
Women who do not ovulate and have irregular periods or absence of menstruation
As a general rule, the first injection of a vial of 75 IU of menotropin is administered during the first week of the cycle after spontaneous or induced menstruation.
Subsequently, the prescribed dose of this medicine is injected daily, and treatment is continued until one or more mature follicles have formed in the ovary. Your doctor will adjust the dose of this medicine based on the ovarian response, which is determined by clinical examinations.
Once a follicle reaches the necessary stage of development, treatment with this medicine will be stopped, and ovulation will be triggered with another hormone (human chorionic gonadotropin, hCG).
Ovulation usually occurs within 32 to 48 hours.
At this stage of treatment, there is a possibility of fertilization. You will be advised to have sexual intercourse every day starting from the day before hCG administration. If pregnancy is not achieved despite ovulation, treatment may be repeated.
Women undergoing ovarian stimulation for multiple follicular development before in vitro fertilization or other assisted reproduction techniques
The purpose of this method is to achieve simultaneous multiple follicular development. Treatment will start on the second or third day of the cycle with injections of 150 to 300 IU of this medicine (1 or 2 vials of Meriofert Kit 150 IU). Your doctor may choose to administer higher doses if necessary. The dose of this medicine injected is higher than that used for natural fertilization. Your doctor will adjust the continuation of treatment on an individual basis.
Once a sufficient number of follicles have developed, treatment with this medicine will be stopped, and ovulation will be triggered by injecting another hormone (human chorionic gonadotropin, hCG).
How to administer Meriofert Kit
This medicine is administered by injection under the skin (subcutaneously) or into the muscle (intramuscularly).
The vials should only be used once, and the injection should be administered immediately after preparation.
After advising and instructing you properly, your doctor may ask you to administer the Meriofert Kit injection yourself.
The first time you do this, your doctor should
- allow you to practice self-administering a subcutaneous injection,
- indicate the areas where the injection can be administered,
- teach you how to prepare the injectable solution,
- explain how to prepare the correct dose for injection.
Before administering the Meriofert Kit injection, read the following instructions carefully.
How to prepare and inject 1 vial of Meriofert Kit
The injection should be prepared just before you are ready to use it, using the pre-filled syringe of solvent (a 9 mg/ml sodium chloride solution in water for injections) included in each pack of Meriofert Kit.
Prepare a clean surface and wash your hands. It is essential that your hands and the items you use are as clean as possible.
Place the following items on the surface:
- two alcohol swabs (not included),
- a vial of Meriofert Kit powder,
- a pre-filled syringe of solvent,
- a needle for preparing the injection,
- a fine needle for subcutaneous injection.
REMEMBER: do not remove the support ring (white piece) from the pre-filled syringe, as it prevents accidental withdrawal of the piston and improves handling of the syringe during injection.
Reconstitution of the injectable solution
Preparation of the injection
- • Remove the cap from the pre-filled syringe; insert the reconstitution needle (long needle) into the syringe.
• Carefully place the syringe on the clean surface.
• Avoid touching the needle.
Prepare the solution for injection

- • Remove the colored plastic cap (light green for the 75 IU presentation and dark green for the 150 IU presentation) from the Meriofert Kit vial by pushing it gently upwards.
• Clean the rubber stopper with the alcohol swab and wait for it to dry.
- • Take the syringe, remove the protective cap from the needle, and insert the needle through the center of the rubber stopper of the Meriofert Kit vial.
- Push the plunger firmly downwards to spray the entire solution over the powder.
• DO NOT SHAKE THE VIAL; gently move it in circles until a clear solution is obtained.
- The medicine usually dissolves immediately.
- • With the needle still inserted, turn the vial upside down.
- Make sure the tip of the needle is below the level of the liquid.
- Carefully pull back on the plunger to draw the entire Meriofert Kit solution into the syringe.
• Check that the reconstituted solution is clear.
When reconstituting more than 1 vial of Meriofert Kit, insert the contents of the first vial back into the syringe and slowly inject it into a second vial after repeating steps 2 to 4.
Subcutaneous injection of the medicine
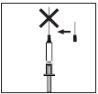
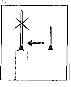 When the syringe contains the prescribed dose, place the protective cap on the needle. Remove the needle from the syringe and replace it with the fine needle for subcutaneous injection with its protective cap.
When the syringe contains the prescribed dose, place the protective cap on the needle. Remove the needle from the syringe and replace it with the fine needle for subcutaneous injection with its protective cap.- Insert the fine needle firmly into the syringe and then twist it slightly to ensure it is fully screwed in and a tight connection is achieved.
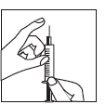
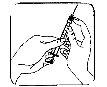 Remove the protective cap from the needle. Hold the syringe with the needle pointing upwards and gently tap the side of the syringe to make any air bubbles rise to the top;
Remove the protective cap from the needle. Hold the syringe with the needle pointing upwards and gently tap the side of the syringe to make any air bubbles rise to the top;- Push the plunger until a drop of liquid appears at the tip of the needle.
- Do not use it if it contains particles or is cloudy.
Injection site
- Your doctor or nurse will have already explained where on your body to administer the medicine. The usual places are the thigh or the lower abdominal wall below the navel.
- Clean the injection site with an alcohol swab.
Insertion of the needle
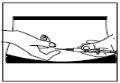
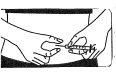
Pinch and firmly press the skin. With the other hand, insert the needle with a quick, firm motion, forming an angle of 45° or 90°.
Injection of the solution
- Inject the syringe under the skin as directed. Do not inject it directly into a vein. Push the plunger slowly and continuously, so the solution is injected correctly and the skin tissues are not damaged.
Take the time you need to inject the prescribed volume of solution. Depending on the dose prescribed by your doctor, you may not use the entire volume of the solution.
Removal of the needle
- Quickly remove the syringe and press the injection site with an antiseptic swab. Gentle massage of the area (while maintaining pressure) helps to disperse the Meriofert Kit solution and alleviate any discomfort.
Intramuscular injection of the medicine
In the case of intramuscular injections, the healthcare professional will prepare and inject this medicine into the side of the thigh or buttock.
Dispose of all used items
Once the injection is complete, place all needles, empty vials, and syringes in the puncture-proof container. The disposal of unused solution and all materials that have come into contact with it will be carried out in accordance with local regulations.
If you use more Meriofert Kit than you should
The effects of overdose of this medicine are unknown; however, it is likely that ovarian hyperstimulation syndrome (OHSS) (see section 4) would occur. If you use more medicine than you should, consult your doctor or nurse.
If you forget to use Meriofert Kit
Use it at the next scheduled time. Do not take a double dose to make up for forgotten doses.
If you stop using Meriofert Kit
Do not stop treatment on your own initiative.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effect is important and will require immediate action if you experience it. You must stop taking this medicine and see a doctor immediately if you experience:
Common: may affect up to 1 in 10 people
- Ovarian hyperstimulation syndrome (symptoms include the formation of ovarian cysts or the enlargement of existing cysts, pain in the lower abdomen, feeling of thirst, and nausea with occasional vomiting, passing small amounts of concentrated urine, and weight gain) (see section 2 for more information).
The following side effects have also been reported:
Very common: may affect more than 1 in 10 people
- Headache
- Abdominal swelling or bloating
Common: may affect up to 1 in 10 people
- Abdominal pain or discomfort
- Pelvic pain
- Back pain
- Feeling of heaviness
- Breast tenderness
- Dizziness
- Hot flashes
- Thirst
- Feeling sick
- Fatigue
- General malaise
- Reactions at the injection site, such as pain and inflammation (more common with IM than with SC injections).
Rare: may affect up to 1 in 1,000 people
- Ovarian torsion (twisting of the ovary that causes severe pain in the lower abdomen)
- Thromboembolism (formation of a blood clot in a blood vessel that breaks loose and travels through the bloodstream to block another vessel).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Meriofert Kit
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Store the vial and the pre-filled syringe of solvent in the outer packaging to protect them from light.
Do not use this medicine after the expiry date stated on the outer packaging, the vial, and the pre-filled syringe of solvent after CAD. If the expiry date is indicated as month/year, the expiry date is the last day of the month indicated.
Use immediately after reconstitution.
Do not use this medicine if you notice that the solution is not transparent. After reconstitution, the solution should be transparent and colorless.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE  point at the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
point at the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Meriofert Kit
The active ingredient is menotropin.
Each vial contains lyophilized powder with 150 IU of human follicle-stimulating hormone (FSH) activity and 150 IU of human luteinizing hormone (LH) activity.
Human menopausal gonadotropin (HMG) is extracted from the urine of post-menopausal women.
Human chorionic gonadotropin (hCG) is added, a hormone extracted from the urine of pregnant women, to contribute to the total LH activity.
If several vials of powder are used, the amount of menotropin in 1 ml of reconstituted solution will be as follows:
Meriofert Kit 150UI powder and solvent for injectable solution | |
Number of vials used | Total amount of menotropin in 1 ml of solution |
1 | 150 IU |
2 | 300 IU |
3 | 450 IU |
The other components are
Vial of powder: lactose monohydrate
Pre-filled syringe with solvent: 9 mg/ml sodium chloride solution.
Appearance of the Product and Package Contents
Powder: white lyophilized cake or powder
Solvent: transparent and colorless solution
Meriofert Kit is presented as powder and solvent for injectable solution.
1 carton contains the following:
- 1 vial with white lyophilized cake or powder
- 1 pre-filled syringe (1 ml) with transparent and colorless solution
- 1 needle for reconstitution and intramuscular injection (long needle)
- 1 needle for subcutaneous injection (short needle)
Supplied in boxes of 1, 5, or 10 cartons.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
IBSA Farmaceutici Italia Srl
Via Martiri di Cefalonia 2
26900 Lodi, Italy
Manufacturer
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Italy
or (for the United Kingdom)
IBSA Pharma Limited
Units 4-6
Colonial Business Park
Colonial Way
Watford D24 4PR
United Kingdom
You can request more information about this medicine by contacting the local representative of the Marketing Authorization Holder:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (Spain)
This medicine is authorized in the Member States of the European Economic Area under the following names (concentrations and pharmaceutical forms are identical in all countries, only trade names differ):
Austria: Meriofert PFS
Belgium: Fertinorm Kit
Bulgaria: Meriofert PFS
Cyprus: Meriofert PFS
Czech Republic: Meriofert Set
Denmark: Meriofert Set
Estonia: Meriofert Set
Finland: Meriofert Set
France: Fertistartkit
Greece: Meriofert
Hungary: Meriofert Kit
Italy: Meriofert
Latvia: Meriofert Set
Lithuania: Meriofert Set
Luxembourg: Fertinorm Kit
Norway: Meriofert Set
Poland: Mensinorm Set
Romania: Meriofert PFS
Slovakia: Meriofert Kit
Spain: Meriofert Kit
Netherlands: Meriofert spuit
United Kingdom: Meriofert PFS
Sweden: Meriofert Set
Date of the last revision of this leaflet: May 2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MERIOFERT KIT 150 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, -Active substance: human menopausal gonadotrophinManufacturer: Angelini Pharma Espana S.L.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.U.Prescription required
Online doctors for MERIOFERT KIT 150 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about MERIOFERT KIT 150 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






