
MAXIDEX 1 mg/ml EYE DROPS IN SUSPENSION


How to use MAXIDEX 1 mg/ml EYE DROPS IN SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
MAXIDEX 1 mg/ml eye drops, suspension
Dexamethasone
Read the package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is MAXIDEX and what is it used for
- What you need to know before you use MAXIDEX
- How to use MAXIDEX
- Possible side effects
- Storage of MAXIDEX
- Contents of the pack and other information
1. What is Maxidex and what is it used for
It is an eye drop suspension that contains the active substance dexamethasone, a potent corticosteroid with anti-inflammatory and anti-allergic properties, mainly reducing the inflammatory response caused by agents of allergic, mechanical, or chemical nature.
Maxidex is indicated for the treatment of non-infectious eye inflammations that respond to corticosteroids, such as: anterior uveitis (inflammation of the uvea or middle layer of the eye), iris inflammation (iritis), ciliary body inflammation (cyclitis), or conjunctival inflammation (iridocyclitis). Corneal inflammation (keratitis): keratitis caused by the Herpes Zoster virus and superficial punctate keratitis (with small points of injury on the cornea). Complement in the treatment of phlyctenular keratoconjunctivitis (inflammation of the cornea and conjunctiva characterized by the presence of small nodules). Spring and allergic conjunctivitis. Corneal injuries caused by a foreign body. Thermal burns of the eyes.
2. What you need to know before you use Maxidex
Do not use Maxidex:
- If you are allergic to dexamethasone or any of the other components of this medicine (listed in section 6).
- If you have or think you have:
- Untreated bacterial eye infection.
- Corneal inflammation (keratitis) caused by herpes simplex or any other eye infection caused by a virus, such as smallpox or chickenpox.
- Tuberculosis that affects the eye.
- Fungal (fungal) eye diseases or untreated eye infections caused by parasites.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Maxidex.
- Only use this medicine in your eye(s).
- This medicine may increase eye pressure, especially if you already have glaucoma or high eye pressure, or a family history, so you should use it under medical control.
- If you use this medicine for a long time, you may:
- Develop high eye pressure and/or glaucoma (with optic nerve damage and decreased visual acuity). You should regularly check the pressure in your eye while using this medicine. Consult your doctor if you have any doubts. The risk of increased intraocular pressure and/or cataract formation induced by corticosteroids is higher in prone patients (e.g., diabetes).
- Develop cataracts. You should visit your doctor frequently.
- Develop Cushing's syndrome and/or suppression of adrenal gland function because the medicine reaches the blood. Consult your doctor if you suffer from swelling and weight gain around the trunk and face, as these are usually the first manifestations of a syndrome called Cushing's syndrome. Adrenal gland function suppression may occur after intensive or long-term treatment with Maxidex. Consult your doctor before stopping treatment on your own. These risks are especially important in children and patients treated with a medicine called ritonavir or cobicistat.
- If your symptoms worsen or return suddenly, please contact your doctor. You may become more sensitive to eye infections with the use of this medicine. Corticosteroids can also mask the signs of an infection or worsen it, especially with prolonged use in eye infections with pus.
- If you already have a bacterial eye infection, you should consult your doctor about your treatment.
- Prolonged use of corticosteroids in the eye could produce fungal infections in the cornea. If this occurs, treatment should be discontinued. The use of corticosteroids in the eye in excessive doses can delay the healing of eye wounds. It is also known that ophthalmic NSAIDs can slow down or delay healing (see section "Other medicines and Maxidex"). If you suffer from a disorder that causes thinning of the eye tissue (the outermost layers of the eye), the use of this medicine could cause corneal perforation.
- Contact your doctor if you experience blurred vision or other visual disturbances.
If you wear contact lenses:
- Wearing contact lenses (hard or soft) is not recommended during the treatment of an eye inflammation.
Children
The safety and efficacy of this medicine in children have not been established. Therefore, its use is not recommended in children.
The possible increase in intraocular pressure associated with the prolonged use of this medicine is especially important in pediatric patients; the risk of corticosteroid-induced ocular hypertension may be higher in children and occur earlier than in adults.
Other medicines and Maxidex
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Tell your doctor if you are using ophthalmic NSAIDs. The concomitant use of steroids and ophthalmic NSAIDs can increase corneal healing problems.
Tell your doctor if you are using ritonavir or cobicistat, as they may cause an increase in the amount of dexamethasone in the blood.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
The use of Maxidex is not recommended during pregnancy.
If you are breastfeeding, you should decide whether to stop breastfeeding or stop treatment with this medicine, taking into account the benefit of breastfeeding for the child and the benefit of treatment for the mother.
Driving and using machines
You may notice that your vision becomes blurred for a while after applying the eye drops. Do not drive or use machines until this effect has disappeared.
Maxidex contains benzalkonium chloride and phosphates
This medicine contains 0.5 mg of benzalkonium chloride in each 5 ml bottle, equivalent to 0.1 mg/ml.
Benzalkonium chloride can be absorbed by soft contact lenses, altering their color. Remove contact lenses before using this medicine and wait 15 minutes before putting them back.
Benzalkonium chloride can cause eye irritation, especially if you have dry eye or other corneal diseases (the transparent layer of the front of the eye). Consult your doctor if you feel a strange sensation, itching, or pain in the eye after using this medicine.
This medicine contains 6.5 mg of phosphates in each 5 ml bottle, equivalent to 1.3 mg/ml.
If you have severe corneal damage (the transparent layer of the front of the eye), treatment with phosphates, in very rare cases, can cause blurred vision due to calcium accumulation.
3. How to use Maxidex
Follow the administration instructions of this medicine exactly as indicated by your doctor. If you have any doubts, consult your doctor or pharmacist again.
Ophthalmic route (in the eye(s)).
The recommended dose is:
Adults:
Usually, instill 1 or 2 drops in the affected eye, 5 or 6 times a day.
When a satisfactory response is observed after 3-4 days, the frequency of administration can be gradually reduced to less than once a day.
The maximum recommended treatment duration is 14 days, unless your doctor has given you different instructions.
Recommendations for use:
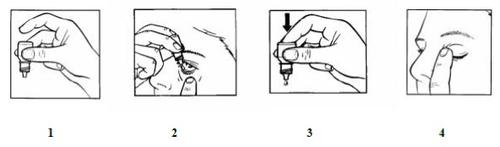
- Wash your hands.
- Take the bottle (dropper bottle) and shake it well before use.
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
- Hold the bottle, upside down, between your fingers (figure 1).
- Tilt your head back. Gently separate the eyelid from the eye with a finger until a pocket forms between the eyelid and your eye, where the drop should fall (figure 2).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, or nearby areas, with the dropper. The drops could become contaminated.
- Gently squeeze the base of the bottle to release one drop at a time (figure 3).
- After using this eye drop, press the edge of the eye next to the nose with your finger. This helps prevent the medicine from passing into the rest of the body (figure 4).
- If you are applying drops in both eyes, repeat all the previous steps with the other eye.
- Close the bottle tightly immediately after using the product.
If a drop falls outside the eye, try again.
If you are using other ophthalmic medicines,wait at least 5 minutes between the administration of this eye drop and the other ophthalmic medicines. Ophthalmic ointments should be administered last.
If you use more Maxidex than you should
An overdose in the eyes can be eliminated by rinsing the eyes with warm water. Do not apply more drops until the next dose.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Maxidex
Do not apply a double dose to make up for forgotten doses.
Apply a single dose as soon as you remember and continue with the next dose that was scheduled. However, if it is almost time for the next dose, do not apply the forgotten dose and continue with the next dose of your usual regimen.
If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects are classified by frequency, which is defined as: very common (may affect more than 1 in 10 people); common (may affect up to 1 in 10 people); uncommon (may affect up to 1 in 100 people); rare (may affect up to 1 in 1,000 people); very rare (may affect up to 1 in 10,000 people); frequency not known (cannot be estimated from the available data).
The following side effects have been reported with this medicine:
Common side effects:
- Eye effects: discomfort in the eye(s).
Uncommon side effects:
- Eye effects: inflammation of the eye surface (keratitis), conjunctivitis, dry eye, corneal spot, sensitivity to light (photophobia), blurred vision, abnormal sensation in the eye, tearing, eyelid crusts, itching, irritation, or redness of the eye.
- General effects: alteration of taste (bad taste).
Frequency not known:
- Eye effects: glaucoma (increased eye pressure with decreased visual acuity), corneal ulcer, increased pressure in the eye(s), reduced vision, corneal damage, eyelid drooping, eye pain, increased pupil size.
- General effects: hypersensitivity (allergy), dizziness, headache.
- Hormonal problems: excessive growth of body hair (especially in women), weakness and muscle wasting, purple striae on the skin of the body, increased blood pressure, irregular or absent menstrual periods, changes in protein and calcium levels in the body, delayed growth in children and adolescents, and swelling and weight gain in the body and face (Cushing's syndrome) (see section 2, "Warnings and precautions").
The prolonged use of corticosteroids in the eyes can also cause:
- Increased eye pressure with optic nerve damage and decreased visual acuity.
- Cataract formation.
- Delayed healing of the cornea.
- In diseases that cause thinning of the cornea, there is a higher risk of perforation.
Corticosteroids can reduce resistance to eye infections, favoring their appearance.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects that are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es.By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Maxidex
Keep this medicine out of the sight and reach of children.
Do not store above 30°C.
Do not use this medicine after the expiry date stated on the bottle and carton after EXP. The expiry date is the last day of the month indicated.
To avoid infections, you should discard the bottle 4 weeks after opening it for the first time.
Write the date of opening of the bottle in the space provided for this purpose on the carton.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If you have any doubts, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and other information
Composition of MAXIDEX
- The active substance is dexamethasone. 1 ml of suspension contains 1 mg of dexamethasone (0.1%).
- The other ingredients are: benzalkonium chloride, anhydrous disodium hydrogen phosphate, polysorbate 80, disodium edetate, sodium chloride, hypromellose, citric acid monohydrate and/or sodium hydroxide, and purified water.
Appearance and packaging of the product
Maxidex is a white or slightly yellowish eye drop suspension. It comes in a dropper bottle (plastic bottle with screw cap).
Each pack contains 5 ml of eye drops.
Marketing authorization holder
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 – Barcelona, Spain
Manufacturer
Siegfried El Masnou, S.A.
C/Camil Fabra, 58
08320 El Masnou – Barcelona, Spain
or
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona, Spain
or
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg, Germany
Date of last revision of this leaflet:January 2021
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price2.5 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MAXIDEX 1 mg/ml EYE DROPS IN SUSPENSIONDosage form: EYEDROP, 1 mg/mlActive substance: dexamethasoneManufacturer: Pharmaselect International Beteiligungs GmbhPrescription requiredDosage form: EYE DROP, 0.1 %Active substance: dexamethasoneManufacturer: Fidia Farmaceutici S.P.A.Prescription requiredDosage form: EYEDROP, 1mg/mlActive substance: dexamethasoneManufacturer: Laboratoires TheaPrescription required
Online doctors for MAXIDEX 1 mg/ml EYE DROPS IN SUSPENSION
Discuss questions about MAXIDEX 1 mg/ml EYE DROPS IN SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















