
LETYBO 50 units powder for injectable solution

How to use LETYBO 50 units powder for injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Letybo 50powder for solution for injection
botulinum toxin type A
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Letybo and what is it used for
- What you need to know before you use Letybo
- How to use Letybo
- Possible side effects
- Storage of Letybo
- Contents of the pack and further information
1. What is Letybo and what is it used for
Letybo contains the active substance botulinum toxin type A. It works by blocking the nerve impulses that go to the muscles into which it has been injected. In this way, it prevents the muscles from contracting, causing a temporary paralysis.
Letybo is used in adults under 75 years to temporarily improve moderate to severe vertical lines between the eyebrows when their presence has a significant psychological impact on these individuals.
2. What you need to know before you use Letybo
Do not use Letybo:
- if you are allergic to botulinum toxin type A or any of the other ingredients of this medicine (listed in section 6)
- if you have a muscle disorder, such as myasthenia gravis, Lambert-Eaton syndrome, or amyotrophic lateral sclerosis
- if you have an acute infection or inflammation in the proposed injection sites
Warnings and precautions
Talk to your doctor before using Letybo if you have:
- any disorder that affects the muscles and/or their direct control by the nervous system
- difficulty swallowing or breathing, or if you have had them before
- a bleeding disorder
If you have a history of these problems, it is not recommended that you use Letybo.
Pain related to needles or fear of injections can cause a feeling of loss of consciousness due to a sudden drop in blood pressure.
Very rarely, side effects caused by the spread of botulinum toxin away from the injection site have been reported, such as excessive muscle weakness. Difficulty swallowing and breathing are serious and can be life-threatening.
If you have trouble swallowing, speaking, or breathing, seek medical help immediately.
Children and adolescents
Letybo is not recommended for children and adolescents under 18 years.
Other medicines and Letybo
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
The following medicines may affect or be affected by Letybo:
- medicines that affect the transmission of nerve impulses to muscles
- some medicines used to treat bacterial infections, such as spectinomycin or aminoglycoside antibiotics
- other medicines that contain botulinum toxin.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Letybo should not be used during pregnancy or breastfeeding, or in women of childbearing potential who are not using contraception.
Driving and using machines
Botulinum toxin type A can cause weakness, dizziness, and visual disturbances. Do not drive or use machines if your ability to react is reduced.
Letybo contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially “sodium-free”.
3. How to use Letybo
This unit of botulinum toxin is specific to Letybo. This means that it is different from other units of botulinum toxin and cannot be exchanged with those used in other botulinum toxin preparations.
Letybo has been prescribed exclusively for you by a doctor who is competent in the use of this treatment and has the correct equipment. A detailed description of the preparation of the solution and the instructions for use are described in the section “This information is intended only for healthcare professionals” at the end of the package leaflet.
The recommended dose is
20 units divided into five injections of 0.1 ml (4 units). Each injection is administered into the muscles located above or between the eyebrows.
Letybo is administered by intramuscular (IM) injection.
Once the solution has been reconstituted, the vial should only be used for one session per patient. Any unused solution should be discarded, as explained after section 6 of the information for healthcare professionals.
It is recommended to wait at least 3 months between two treatments with Letybo.
If you have been given more Letybo than you should
Overdoses can cause muscle and/or nerve paralysis. The signs of overdose may not appear immediately after injection.
In case of overdose, your doctor will monitor you for symptoms, such as general weakness or muscle paralysis. You will be hospitalized if you show symptoms of botulinum toxin type A poisoning, such as:
- generalized weakness
- drooping of the upper eyelid or double vision
- speech or swallowing disorders
- partial paralysis of the muscles that control breathing.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects are mild to moderate, occur in the first few days after injection, and are transient.
Some side effects can be very serious. If you experience any of the following side effects, tell your doctor immediately or ask your family members to tell your doctor and go to the nearest emergency department:
Uncommon, may affect 1 in 100 people
- drooping of the upper eyelid, eyelid spasm
Rare, may affect 1 in 1,000 people
- eyelid sensory disorder, eyebrow drooping
- conjunctival bleeding
- eye pain, dry eye, visual field defect, blurred vision
- reduced sensitivity in the throat
- constipation
- joint disorder
Very rare, may affect 1 in 10,000 people
- muscle weakness
- difficulty swallowing
- respiratory or lung infection caused by aspiration of food or liquid
- difficulty breathing
In addition to these possible side effects, a severe allergic reaction could cause the following symptoms:
- difficulty swallowing, breathing, or speaking due to swelling in the face, lips, mouth, or throat; in addition to these symptoms, hives may occur (see section 2)
Other known side effects may occur with the following frequencies. Tell your doctor or pharmacist if they are severe:
Common, may affect 1 in 10 people
- headache
- reactions at the injection site
Uncommon, may affect 1 in 100 people
- head discomfort
- local swelling, e.g., in the eyelid, face, or around the eyes
- pain, bruising, swelling, itching, increased volume, or pressure at the injection site
- bruising, e.g., around the eyes
- infection, such as an upper respiratory tract viral infection, e.g., cold
- Mephisto effect (lateral elevation of the eyebrows)
Rare, may affect 1 in 1,000 people
- migraine
- folliculitis
- dizziness
- abnormal sensations, such as pins and needles, tingling, and itching
- nausea
- dry skin, rash, itching
- facial pain
- fever
- oral herpes
- high blood potassium
- pseudogrippal syndrome
Reporting of side effects
If you experience side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Surveillance System for Human Use, https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Letybo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after “EXP”. The expiry date refers to the last day of the month shown.
Store and transport refrigerated (2°C – 8°C).
Reconstituted solution
Chemical and physical stability has been demonstrated for 24 hours at 2°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user and normally should not exceed 24 hours at a temperature between 2 and 8°C, unless the reconstitution/dilution (etc.) has been carried out under controlled and validated aseptic conditions.
Any unused solution should be discarded after 24 hours.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Letybo
- The active substance is botulinum toxin type A.
- One vial contains 50 units of botulinum toxin type A produced by Clostridium botulinum.
- After reconstitution, the solution contains 4 units per 0.1 ml.
- The other excipients are human albumin, sodium chloride.
Appearance and pack contents
Letybo is a white powder for solution for injection supplied in a transparent glass vial with a rubber stopper and an aluminum flip-off cap.
The individual pack contains 1 or 2 vials.
The multiple pack contains 2 boxes, each with 1 vial.
The multiple pack contains 6 boxes, each with 1 vial.
Not all pack sizes may be marketed.
Marketing authorisation holder
CROMA-PHARMA GmbH
Industriezeile 6
2100 Leobendorf
Austria
Manufacturer
Croma-Pharma GmbH
Cromazeile 2
2100 Leobendorf
Austria
Date of last revision of this leaflet:July 2023
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑
This information is intended only for healthcare professionals:
The units of botulinum toxin cannot be exchanged from one product to another. The recommended doses in units are different from those of other botulinum toxin preparations.
The instructions for use, handling, and disposal must be followed strictly.
Preparation of the solution
Reconstitution should be performed in accordance with good practice, especially with regard to asepsis.
For reconstitution of Letybo, a 9 mg/ml (0.9%) sodium chloride injectable solution should be used as a diluent and should be added to a volume of 1.25 ml.
Best practice is to reconstitute the vial contents and prepare the syringe on plastic-coated paper towels to catch any spill. The 9 mg/ml (0.9%) sodium chloride injectable solution is drawn into the syringe and should be slowly injected into the vial to avoid foaming/bubbling or vigorous agitation that could cause denaturation. The vial should be discarded if the vacuum does not draw the solvent into the vial. Once reconstituted, Letybo is a clear, colorless solution, free from particulates. Before use, a visual inspection of the vial should be performed to ensure that the product is free from foreign particles.
Letybo should not be used if the reconstituted solution has a cloudy appearance or contains particulates.
Reconstituted solution
Chemical and physical stability has been demonstrated for 24 hours at 2°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user and normally should not exceed 24 hours at a temperature between 2 and 8°C, unless the reconstitution/dilution (etc.) has been carried out under controlled and validated aseptic conditions.
Any solution that has been stored for more than 24 hours should be discarded.
The disposal of unused medicinal products and all materials that have come into contact with them should be carried out in accordance with local requirements.
Instructions for use
Intramuscular injections should be performed with a sterile insulin or tuberculin syringe of 1 ml, with a graduation of 0.01 ml and a 30-31 G needle.
A volume of 0.5 ml of the reconstituted Letybo solution should be drawn into the sterile syringe and any air bubbles present in the syringe barrel should be expelled. The needle used to reconstitute the medicinal product should be removed and replaced with another for administration.
Care should be taken not to inject Letybo into a blood vessel.
To reduce complications due to blepharoptosis, injections near the levator palpebrae superiorisshould be avoided, especially in patients with a large eyebrow-depressor complex. When injecting into two areas of each corrugator superciliimuscle, the first injection should be made just above the middle margin of the eyebrows. The second injection should be made approximately 1 cm above the supraorbital ridge (palpable rigid bony limits above the upper eyelid), where the medial lines of the eyebrows meet. The injection site of the procerusmuscle is just above the midline of the nasal bridge, where horizontal wrinkles form between the medial ends of the eyebrows. When injecting into the medial ends of the corrugator superciliimuscles and the medial lines of the eyebrows, the injection sites should be at least 1 cm away from the supraorbital ridge (palpable rigid bony limits above the upper eyelid).
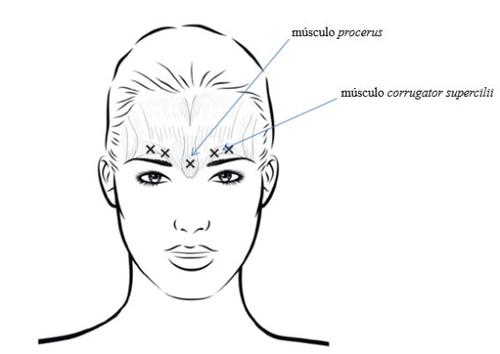
Injections should be administered carefully to avoid intravascular injection. Before injection, the thumb or index finger can be placed firmly under the supraorbital ridge to prevent the medicinal product from spilling into that area. The needle should be directed upwards and medially.
In case of failed treatment one month after a previous session, i.e., in the absence of significant improvement from the start, the following strategies can be considered:
- Analysis of the causes of failure; e.g., injection into the wrong muscles, injection technique, formation of neutralizing antibodies to the toxin, insufficient dose
- Re-evaluation of whether treatment with botulinum toxin type A is appropriate
In the absence of unwanted side effects as a result of a previous treatment session, it is possible to initiate another treatment session with a minimum interval of three months between treatment sessions.
Procedure to be followed for the safe disposal of vials, syringes, and materials used
For safe disposal, unreconstituted Letybo should be reconstituted in the vial with a small amount of water and then sterilized by autoclaving. Empty vials, vials containing residual solution, syringes, or spills should be sterilized by autoclaving. Alternatively, residual Letybo can be inactivated with a diluted sodium hydroxide solution (0.1 N NaOH) or a diluted sodium hypochlorite solution (0.5% or 1% NaOCl).
After inactivation, vials, syringes, and materials used should not be emptied and should be disposed of in appropriate containers and eliminated in accordance with local regulations.
Recommendations in case of an incident during the handling of botulinum toxin
- Any spill of the product should be cleaned up: with absorbent material impregnated with a sodium hypochlorite solution in the case of the powder, or with dry absorbent material in the case of the reconstituted product.
- Contaminated surfaces should be cleaned with absorbent material impregnated with a sodium hypochlorite solution and then dried.
- If a vial is broken, proceed as indicated above, carefully collecting the broken glass and cleaning the product. Avoid cutting your skin.
- If the medicinal product comes into contact with the skin, wash the affected area with a sodium hypochlorite solution and rinse with plenty of water.
- If the medicinal product comes into contact with the eyes, wash them thoroughly with plenty of water or with an ophthalmic washing solution.
If the medicinal product comes into contact with a wound or broken skin, wash the area thoroughly with plenty of water and take appropriate medical measures according to the injected dose.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LETYBO 50 units powder for injectable solutionDosage form: INJECTABLE, 200 U/mlActive substance: botulinum toxinManufacturer: Ipsen PharmaPrescription requiredDosage form: INJECTABLE, 125 Speywood UnitsActive substance: botulinum toxinManufacturer: Ipsen Pharma S.A.U.Prescription requiredDosage form: INJECTABLE, 100 unitsActive substance: botulinum toxinManufacturer: Merz Pharmaceuticals GmbhPrescription required
Online doctors for LETYBO 50 units powder for injectable solution
Discuss questions about LETYBO 50 units powder for injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















