
IZAMBY 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use IZAMBY 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Izamby 60 mg solution for injection in pre-filled syringe
denosumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- Your doctor will provide you with a patient reminder card, which contains important safety information that you should know before and during treatment with Izamby.
Contents of the pack
- What is Izamby and what is it used for
- What you need to know before you use Izamby
- How to use Izamby
- Possible side effects
- Storage of Izamby
- Contents of the pack and other information
1. What is Izamby and what is it used for
What is Izamby and how does it work
Izamby contains denosumab, a protein (monoclonal antibody) that interferes with the action of another protein to treat bone loss and osteoporosis. Treatment with denosumab strengthens bones and reduces the risk of fractures.
Bone is a living tissue that is constantly renewed. Estrogens contribute to the preservation of bone health. After menopause, estrogen levels decrease, which can cause bones to become thinner and more fragile. Over time, this can lead to a disease called osteoporosis. Osteoporosis can also occur in men due to various causes, including age and/or low levels of the male hormone, testosterone. It can also occur in patients undergoing treatment with glucocorticoids. Many patients with osteoporosis do not have symptoms, although they still have a risk of fracturing bones, especially in the spine, hip, and wrists.
Surgical interventions or medications that stop the production of estrogen or testosterone, used to treat patients with prostate or breast cancer, can also cause bone loss. This makes bones weaker and more prone to breaking.
What is Izamby used for
Izamby is used to treat:
- postmenopausal osteoporosis in women and men who have an increased risk of fracture (bone breakage), reducing the risk of fractures of the hip, spine, and non-spinal locations,
- bone loss caused by reduced hormonal levels (testosterone) as a result of surgical intervention or treatment with medications in patients with prostate cancer,
- bone loss resulting from long-term treatment with glucocorticoids in patients who have a high risk of fracture.
2. What you need to know before you use Izamby
Do not use Izamby
- if you have low levels of calcium in the blood (hypocalcemia),
- if you are allergic to denosumab or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Izamby.
During treatment with Izamby, you may develop a skin infection with symptoms such as an inflamed and reddened area on the skin, more frequently on the lower leg, which feels hot and sensitive to the touch (cellulitis), and may be accompanied by fever. Inform your doctor immediately if you experience any of these symptoms.
In addition, you should take calcium and vitamin D supplements during treatment with Izamby. Your doctor will discuss this with you.
While receiving Izamby, you may experience low levels of calcium in the blood. Inform your doctor immediately if you notice any of the following symptoms: muscle spasms, contractions, or cramps, and/or numbness or tingling in the fingers of the hands, feet, or around the mouth, and/or convulsions, confusion, or loss of consciousness.
In rare cases, very low levels of calcium in the blood have been reported, which have required hospitalization and, in some cases, have led to potentially life-threatening reactions. Therefore, before each dose is administered and, in patients with a predisposition to hypocalcemia, within two weeks after the initial dose, your blood calcium levels will be checked (through a blood test).
Inform your doctor if you have or have had severe kidney problems, kidney failure, if you have needed to undergo dialysis, or if you are taking medications called glucocorticoids (such as prednisolone or dexamethasone), as they may increase the risk of having low levels of calcium in the blood if you do not take calcium supplements.
Problems in the mouth, teeth, or jaw
In patients receiving denosumab for osteoporosis, a rare side effect called osteonecrosis of the jaw (ONJ) (damage to the jawbone) has been reported (may affect up to 1 in 1,000 people). The risk of ONJ increases in patients treated for a long time (may affect up to 1 in 200 people if treated for 10 years). ONJ can also occur after stopping treatment. It is essential to try to prevent the development of ONJ, as it can be a painful condition that can be difficult to treat. To reduce the risk of developing ONJ, follow these precautions:
Before receiving treatment, inform your doctor or nurse (healthcare professional) if:
- you have any problems in your mouth or teeth, such as poor dental health, gum disease, or a planned tooth extraction,
- you do not receive regular dental check-ups or have not had a dental check-up for a long time,
- you are a smoker (as this may increase the risk of dental problems),
- you have been previously treated with a bisphosphonate (used to prevent or treat bone disorders),
- you are taking medications called corticosteroids (such as prednisolone or dexamethasone),
- you have cancer.
Your doctor may ask you to undergo a dental check-up before starting treatment with Izamby.
During treatment, you should maintain good oral hygiene and undergo routine dental check-ups. If you use dental prosthetics, ensure they fit properly. If you are undergoing dental treatment or are going to undergo dental surgery (e.g., tooth extractions), inform your doctor about your dental treatment and inform your dentist that you are being treated with Izamby.
Contact your doctor and dentist immediately if you experience any problems in your mouth or teeth, such as loose teeth, pain or inflammation, or ulcers that do not heal or are suppurating, as these could be symptoms of ONJ.
Unusual fractures of the thigh
Some people have developed unusual fractures in the thigh while being treated with denosumab. Consult your doctor if you experience new or unusual pain in the hip, groin, or thigh.
Children and adolescents
Izamby should not be used in children and adolescents under 18 years of age.
Other medicines and Izamby
Inform your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. It is especially important that you inform your doctor if you are being treated with another medicine that contains denosumab.
Do not use Izamby with another medicine that contains denosumab.
Pregnancy and breastfeeding
Izamby has not been tested in pregnant women. If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. Izamby is not recommended during pregnancy. Women of childbearing age should use effective contraceptive methods during treatment with Izamby and for at least 5 months after stopping treatment with Izamby.
If you become pregnant during treatment with Izamby or less than 5 months after stopping treatment with Izamby, inform your doctor.
It is not known if Izamby is excreted in breast milk. It is essential that you inform your doctor if you are breastfeeding or plan to breastfeed. Your doctor will help you decide whether to stop breastfeeding or stop using Izamby, considering the benefit of breastfeeding for the child and the benefit of Izamby for the mother.
If you are breastfeeding during treatment with Izamby, please inform your doctor.
Consult your doctor or pharmacist before using any medicine.
Driving and using machines
The influence of Izamby on the ability to drive and use machines is negligible.
Izamby contains sorbitol
This medicine contains 46 mg of sorbitol in each ml of solution.
Izamby contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 60 mg; this is essentially "sodium-free".
Izamby contains polysorbate
This medicine contains 0.1 mg of polysorbate 20 (E 432) in each pre-filled syringe, equivalent to 0.1 mg/ml.
Polysorbates can cause allergic reactions. Inform your doctor if you have any known allergies.
3. How to use Izamby
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are unsure, consult your doctor again.
The recommended dose is a pre-filled syringe of 60 mg administered under the skin (subcutaneously) in a single injection once every 6 months. The best places for injection are the upper thighs and abdomen. If the injection is given by a caregiver (the person who takes care of you), the injection can also be administered in the outer aspect of the upper arm. Consult your doctor for the date of the next possible injection. Each pack of Izamby contains a reminder card that can be used to keep track of the date of the next injection.
In addition, you should take calcium and vitamin D supplements during treatment with Izamby. Your doctor will discuss this with you.
Your doctor may decide whether it is better for the injection of Izamby to be administered by you or a caregiver. Your doctor or healthcare professional will show you or your caregiver how to use Izamby. If you want to obtain instructions on how to inject Izamby, read the last section of this leaflet.
Do not shake.
Before administration, the solution should be inspected. Do not inject the solution if it contains particles, is cloudy, or has changed color.
If you miss a dose of Izamby
If you miss a dose of Izamby, the injection should be administered as soon as possible. Subsequently, injections should be scheduled every 6 months from the date of the last injection.
If you stop treatment with Izamby
To get the most benefit from your treatment and reduce the risk of fractures, it is essential that you use Izamby for the entire period prescribed by your doctor. Do not stop treatment without talking to your doctor first.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Patients treated with Izamby may develop skin infections (mainly cellulitis) infrequently. Tell your doctor immediatelyif you experience any of these symptoms during treatment with Izamby: an inflamed and reddened area on the skin, usually on the lower leg, which feels hot and sensitive to the touch and may be accompanied by fever.
Rarely, patients receiving Izamby may develop pain in the mouth and/or jaw, inflammation, or ulcers that do not heal in the mouth or jaw, suppurating, numbness, or a feeling of heaviness in the jaw, or tooth mobility. These could be symptoms of bone damage in the jaw (osteonecrosis). Tell your doctor and dentist immediatelyif you experience such symptoms while being treated with Izamby or after stopping treatment.
Rarely, patients receiving Izamby may experience low levels of calcium in the blood (hypocalcemia); very low levels of calcium in the blood may require hospitalization and, in some cases, may be life-threatening. Symptoms include muscle spasms, contractions, or cramps, and/or numbness or tingling in the fingers of the hands, feet, or around the mouth, and/or convulsions, confusion, or loss of consciousness. If you experience any of these, tell your doctor immediately. Low levels of calcium in the blood can also cause a change in heart rhythm called QT prolongation, which can be seen on an electrocardiogram (ECG).
Rarely, unusual fractures of the thigh may occur in patients receiving Izamby. Consult your doctorif you experience new or unusual pain in the hip, groin, or thigh, as this may be an early indication of a possible fracture of the thigh.
Rarely, allergic reactions may occur in patients receiving Izamby. Symptoms include swelling in the face, lips, tongue, throat, or other parts of the body; rash, itching, or hives on the skin; wheezing or difficulty breathing. Tell your doctorif you experience such symptoms while being treated with Izamby.
Very common side effects(may affect more than 1 in 10 people):
- bone, joint, and/or muscle pain, which can be intense,
- pain in the legs or arms (pain in the extremities).
Common side effects(may affect up to 1 in 10 people):
- painful urination, frequent urination, presence of blood in the urine, urinary incontinence,
- upper respiratory tract infection,
- pain, numbness, or tingling that extends to the lower leg (sciatica),
- constipation,
- abdominal discomfort,
- skin rash,
- skin condition with itching, redness, and/or dryness (eczema),
- hair loss (alopecia).
Uncommon side effects(may affect up to 1 in 100 people):
- fever, vomiting, and abdominal pain or discomfort (diverticulitis),
- ear infection,
- skin rash or mouth ulcers (drug-induced lichenoid eruptions).
Rare side effects(may affect up to 1 in 10,000 people):
- allergic reaction that can damage blood vessels, mainly in the skin (e.g., purple or reddish-brown spots, hives, or skin ulcers) (hypersensitivity vasculitis).
Frequency not known(cannot be estimated from the available data):
- consult your doctor if you have ear pain, your ear is suppurating, and/or you have an ear infection. These could be symptoms of damage to the bones of the ear.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Izamby
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after "EXP". The expiry date refers to the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Keep the pre-filled syringe in the outer packaging to protect it from light.
Before injection, the pre-filled syringe can be left outside the refrigerator to reach room temperature (up to 25°C). This will make the injection less painful. Once the pre-filled syringe has reached room temperature (up to 25°C), it must be used within 30 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Izamby Composition
- The active ingredient is denosumab. Each 1 ml pre-filled syringe contains 60 mg of denosumab (60 mg/ml).
- The other components are glacial acetic acid, sodium hydroxide, sorbitol (E 420), polysorbate 20, and water for injectable preparations.
Product Appearance and Container Contents
Izamby is a colorless to slightly yellowish injectable liquid, available in a pre-filled syringe ready for use.
Each container contains a pre-filled syringe with a needle protector.
Marketing Authorization Holder
Mabxience Research SL
C/ Manuel Pombo Angulo 28
28050 Madrid
Spain
Manufacturer
GH GENHELIX S.A.
Parque Tecnológico de León
Edificio GENHELIX
C/Julia Morros, s/n
Armunia, 24009 León Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Date of the Last Revision of this Prospectus:<{MM/AAAA}><{mes AAAA}>.
België/Belgique/Belgien Mabxience Research SL Tél/Tel: + 34 917 711 500 | Lietuva UAB EGIS Lithuania Tel: + 370 5 231 4658 |
| Luxembourg/Luxemburg Mabxience Research SL Tél/Tel: + 34 917 711 500 |
Ceská republika EGIS Praha, spol. s r.o Tel: + 420 227 129 111 | Magyarország Egis Gyógyszergyár Zrt. Tel.: + 36 1 803 5555 |
Danmark Medical Valley Invest AB Tlf: + 46 40 122131 | Malta Mabxience Research SL Tel: + 34 917 711 500 |
Deutschland Mabxience Research SL Tel: + 34 917 711 500 | Nederland Medical Valley Invest AB Tel: + 46 40 122131 |
Eesti Mabxience Research SL Tel: + 34 917 711 500 | Norge Medical Valley Invest AB Tlf: + 46 40 122131 |
Ελλáδα ELPEN Pharmaceutical Co. Inc Τel: + 30 210 6039326-9 | Österreich Gebro Pharma GmbH Tel: + 43 (0)5453 5300-0 |
España Laboratorios Gebro Pharma S.A. Tel: + 34 93 205 86 86 | Polska Egis Polska sp. z o.o. Tel.: + 48 22 417 92 00 |
France Laboratoires Biogaran Tél: + 33 (0) 800 970 109 | Portugal Mabxience Research SL Tel: + 34 917 711 500 |
Hrvatska Mabxience Research SL Tel: + 34 917 711 500 | România Egis Rompharma SRL Tel: + 40 21 412 00 17 |
Ireland Mabxience Research SL Tel: + 34 917 711 500 | Slovenija Mabxience Research SL Tel: + 34 917 711 500 |
Ísland Mabxience Research SL Sími: + 34 917 711 500 | Slovenská republika EGIS SLOVAKIA spol. s r.o., Tel: + 421 2 3240 9422 |
Italia Abiogen Pharma S.p.A Tel: + 39 050 315 4101 | Suomi/Finland Medical Valley Invest AB Puh/Tel: + 46 40 122131 |
Κúπρος Mabxience Research SL Τηλ: + 34 917 711 500 | Sverige Medical Valley Invest AB Tel: + 46 40 122131 |
Latvija Egis Latvia SIA Tel: + 371 676 13859 |
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: https://www.ema.europa.eu.
Instructions for Use
Read these instructions before starting to use the Izamby pre-filled syringes with a needle protector and each time you receive a new package. There may be new information. You should also talk to your healthcare professional about your disease or treatment.
Keep these instructions for use to be able to read them again if necessary.
IMPORTANT INFORMATION
Important information you should know before injecting Izamby:
- It is essential that you do not attempt to administer the injection yourself unless you have received training on the correct way to inject Izamby from your doctor or healthcare professional.
- Izamby is for subcutaneous injection only (inject directly under the skin).
- Do notopen the box until you are ready to use the medication.
- Do notremove the needle cap from the pre-filled syringe until you are ready for injection.
- Do notuse the pre-filled syringe if it has been dropped onto a hard surface. Use a new pre-filled syringe and contact your doctor or healthcare professional.
- Do notattempt to activate the pre-filled syringe before injection.
- Do notattempt to remove the needle protector from the pre-filled syringe.
Contact your doctor or healthcare professional if you or your caregiver have any doubts about the correct way to inject Izamby.
Figure 1 shows the appearance of the pre-filled syringe with a needle protector before (a) and after (b) use.
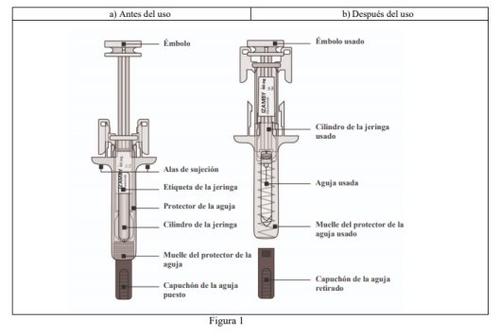
- Prepare for Izamby Injection
Gather Materials
- Place the necessary materials for your injection on a clean and well-lit surface:
- Izamby box with the pre-filled syringe
- Alcohol wipes
- Cotton balls or gauze
- Adhesive bandage
- Sharps container
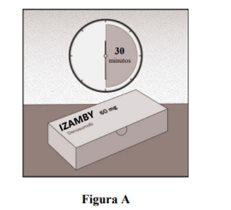
Let it Reach Room Temperature
- To make the injection more comfortable, leave the box with the pre-filled syringe inside at room temperature for about 30 minutes before the injection (Figure A).
- Do notattempt to heat the pre-filled syringe using a heat source such as hot water or a microwave.
- Do notleave the pre-filled syringe exposed to direct sunlight.
- Do notshake the pre-filled syringe.
- Keep the pre-filled syringe out of the sight and reach of children.
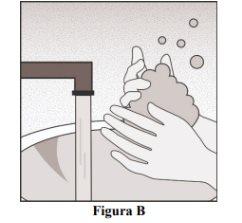
Wash Your Hands
- Wash your hands well with soap and water (Figure B).
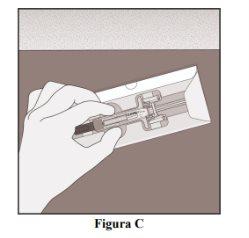
Remove the Pre-filled Syringe from the Box
- Open the box.
- Hold the pre-filled syringe by the body (Figure C).
- Lift the syringe to remove it from the box.
- Place the syringe on a clean and flat surface.
For safety reasons:
- Do nothold it by the plunger.
- Do nothold it by the needle cap.
Examine the Medication and Pre-filled Syringe
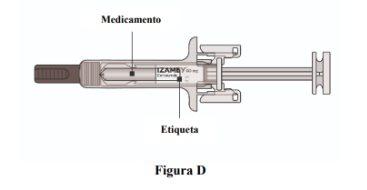
- Check that the product name "Izamby" is printed on the label (Figure D).
- Check the expiration date printed on the label (Figure D).
- Check that the medication is a clear solution, colorless to slightly yellowish (Figure D).
- Check that the pre-filled syringe is not damaged.
Do notuse the pre-filled syringe if:
- The medication is cloudy or contains particles.
- Any of the components are cracked or broken.
- The needle cap is missing or loose.
- The last day of the month indicated on the expiration date printed on the label has passed.
In any of these cases, contact your doctor or healthcare professional.
- Prepare
Prepare the Injection Site
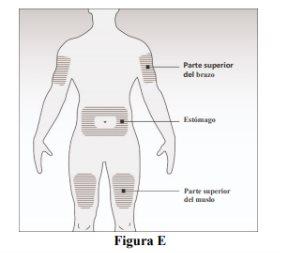
- Choose the injection site (Figure E):
It can be:
-The top of the thigh.
-The stomach, except for an area of 5 cm around the navel.
-The outer side of the upper arm (only if the injection is administered by another person).
Do not inject into areas where the skin is sensitive, bruised, red, or hardened. Avoid injecting into areas with scars or stretch marks.
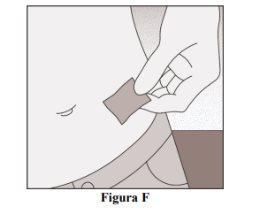
Clean the Injection Site
- Clean the injection site with an alcohol wipe (Figure F).
- Let the skin dry.
- Do not touch the injection site before injecting.
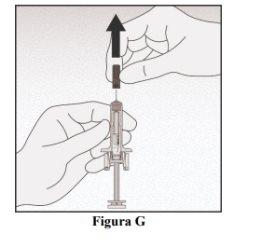
Remove the Needle Cap
- Carefully pull the needle cap straight off and away from your body (Figure G).
- Discard the needle cap.
- Do not attempt to put the needle cap back on the needle.
- Inject Izamby
Insert the Needle
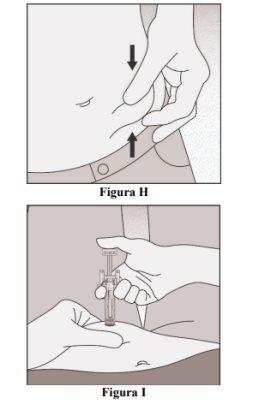
- Pinch the injection site to create a firm surface (Figure H).
- Do not touch the clean area of the skin.
- Note: It is essential to keep the skin pinched when injecting.
- Insert the needle at a 45 to 90-degree angle into the pinched skin (Figure I).
Inject Izamby
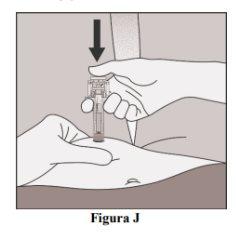
- Slowly press the plunger all the way down until all the liquid has been injected and the syringe is empty (Figure J).
Note:The plunger must be pressed all the way down to ensure that the entire dose is injected and that the needle protector is activated.
Lift Your Thumb
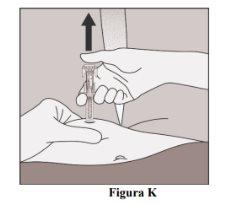
- Lift your thumb off the plunger so that the needle protector covers the needle (Figure K).
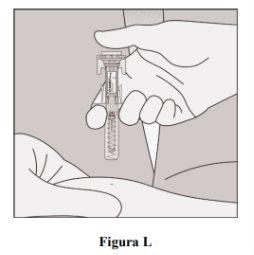
- Remove the needle from the skin (Figure L).
- Release the skin.
Contact your doctor or healthcare professional immediately if:
- the full dose has not been injected or
- the needle protector does not activate after injection.
- Dispose of Izamby
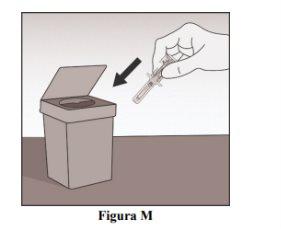
Dispose of the Syringe
- Dispose of the used pre-filled syringe and other materials in a sharps container (Figure M).
- Note:The disposal of medications should be carried out according to local regulations. Ask your doctor or healthcare professional how to dispose of medications that are no longer needed. This will help protect the environment.
- Do notput the needle cap back on used pre-filled syringes.
- Do notreuse the pre-filled syringe even if not all the medication has been injected.
- Do notrecycle the pre-filled syringes or throw them in the trash.
- Keep the syringe and sharps container out of the sight and reach of children.
Examine the Injection Site
- If you see blood, press the injection site with a cotton ball or gauze.
- Do notrub the injection site. If necessary, apply an adhesive bandage.
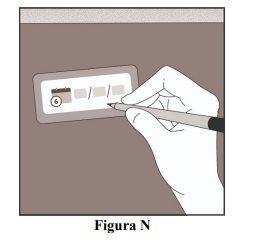
Record the Date of the Next Injection
- Record the date of the next injection on the reminder card included in the package (Figure N).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IZAMBY 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription requiredDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription requiredDosage form: INJECTABLE, 60 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription required
Online doctors for IZAMBY 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about IZAMBY 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













