COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSION INYECTABLE

Cómo usar COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Comirnaty 3 microgramos/dosis concentrado para dispersión inyectable
Lactantes y niños de entre 6 meses y 4 años
vacuna de ARNm frente a COVID-19 (con nucleósidos modificados)
tozinamerán
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera su hijo tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de recibir esta vacuna, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si su hijo experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Comirnaty y para qué se utiliza
- Qué necesita saber antes de que su hijo empiece a recibir Comirnaty
- Cómo se administra Comirnaty
- Posibles efectos adversos
- Conservación de Comirnaty
- Contenido del envase e información adicional
1. Qué es Comirnaty y para qué se utiliza
Comirnaty es una vacuna que se utiliza para prevenir la COVID-19 causada por el SARS-CoV-2.
Comirnaty 3 microgramos/dosis concentrado para dispersión inyectable se administra a lactantes y niños de entre 6 meses y 4 años de edad.
La vacuna hace que el sistema inmunitario (las defensas naturales del organismo) produzca anticuerpos y células sanguíneas que combaten el virus, proporcionando así protección frente a la COVID-19.
Debido a que Comirnaty no contiene el virus para producir inmunidad, no puede causarle a su hijo la COVID-19.
2. Qué necesita saber antes de que su hijo empiece a recibir Comirnaty
Comirnaty no se debe administrar
- si su hijo es alérgico al principio activo o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de que su hijo reciba la vacuna si su hijo:
- ha tenido alguna vez una reacción alérgica grave o problemas para respirar después de la inyección de cualquier otra vacuna o después de que se le administrara Comirnaty en el pasado;
- está nervioso por el proceso de vacunación o se ha desmayado alguna vez después de una inyección con una aguja;
- tiene una enfermedad grave o una infección con fiebre alta. No obstante, su hijo puede ser vacunado si tiene una fiebre leve o una infección de las vías respiratorias altas como un resfriado;
- tiene un problema hemorrágico, se le forman cardenales con facilidad o usa un medicamento para prevenir la formación de coágulos de sangre;
- tiene un sistema inmunitario debilitado debido a una enfermedad como la infección por el VIH o por algún medicamento, como los corticosteroides, que afectan al sistema inmunitario.
Existe un mayor riesgo de miocarditis (inflamación del músculo cardiaco) y pericarditis (inflamación del revestimiento externo del corazón) después de la vacunación con Comirnaty (ver sección 4). Estos trastornos pueden aparecer a los pocos días de la vacunación y se han producido principalmente en un plazo de 14 días. Se han observado con mayor frecuencia tras la segunda dosis de la vacunación, y con mayor frecuencia en varones jóvenes. El riesgo de miocarditis y pericarditis parece ser menor en niños de entre 5 y 11 años de edad que entre los 12 y los 17 años de edad. Después de la vacunación, debe estar alerta a los signos de miocarditis y pericarditis, como dificultad para respirar, palpitaciones y dolor torácico, y debe buscar atención médica inmediata en caso de que aparezcan.
Como con cualquier vacuna, Comirnaty puede no proteger completamente a todas las personas que lo reciban y no se sabe cuánto tiempo estará usted protegido.
La eficacia de Comirnaty, incluso después de una tercera dosis, puede ser menor en personas inmunocomprometidas. En estos casos, debería continuar manteniendo las precauciones físicas para ayudar a prevenir la COVID-19. Además, sus contactos estrechos deberían vacunarse según proceda. Comente con su médico las recomendaciones individuales apropiadas.
Niños
No se recomienda utilizar Comirnaty 3 microgramos/dosis concentrado para dispersión inyectable en niños menores de entre 5 y 11 años de edad. Hay disponible una presentación pediátrica para niños de entre 5 y 11 años de edad. Para más información, consulte el prospecto de Comirnaty 10 microgramos/dosis concentrado para dispersión inyectable.
No se recomienda utilizar Comirnaty en lactantes menores de 6 meses de edad.
Otros medicamentos y Comirnaty
Informe a su médico o farmacéutico si su hijo está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento o ha recibido recientemente cualquier otra vacuna.
Embarazo y lactancia
Comirnaty 3 microgramos/dosis concentrado para dispersión inyectable no está indicado en personaa mayores de 5 años de edad.
Para información detallada sobre el uso en personas mayores de 5 años de edad, consultar el prospecto de Comirnaty 30 microgramos/dosis concentrado para dispersión inyectable, de Comirnaty 30 microgramos/dosis dispersión inyectable o de Comirnaty 10 microgramos/dosis concentrado para dispersión inyectable.
Conducción y uso de máquinas
Algunos de los efectos de la vacunación mencionados en la sección 4 (Posibles efectos adversos) pueden afectar temporalmente a la capacidad para utilizar máquinas o realizar actividades tales como montar en bicicleta. Espere a que estos efectos hayan desaparecido antes de reanudar actividades que requieran su plena atención.
3. Cómo se administra Comirnaty
Comirnaty se administra tras la dilución en forma de inyección de 0,2 ml en un músculo del muslo en los lactantes de entre 6 y menos de 12 meses de edad. En los lactantes y niños de 1 año de edad y mayores, Comirnaty se administra tras dilución en forma de inyección de 0,2 ml en un músculo del muslo o en un músculo del brazo.
Su hijo recibirá tres inyecciones.
Se recomienda recibir la segunda dosis de la misma vacuna 3 semanas después de la primera dosis seguida de una tercera dosis al menos 8 semanas después de la segunda dosis para completar la pauta de vacunación.
Si un niño cumple 5 años de edad entre sus dosis de la pauta de vacunación, deberá completar la pauta con el mismo nivel de dosis de 3 microgramos.
Si tiene cualquier otra duda sobre el uso de Comirnaty, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todas las vacunas, Comirnaty puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- irritabilidad (entre 6 meses y <2 años)
- lugar de inyección: dolor/dolor a la palpación, hinchazón
- cansancio
- dolor de cabeza
- somnolencia (entre 6 meses y <2 años)
- dolor muscular
- escalofríos
- dolor en las articulaciones
- diarrea
- fiebre
Efectos adversos frecuentes:pueden afectar hasta 1 de cada 10 personas
- náuseas
- vómitos
- enrojecimiento en el lugar de inyección («muy frecuente» en niños de entre 6 meses y 11 años de edad)
Efectos adversos poco frecuentes:pueden afectar hasta 1 de cada 100 personas
- aumento de tamaño de los ganglios linfáticos (observado con mayor frecuencia después de la dosis de refuerzo)
- malestar
- dolor en el brazo
- insomnio
- picor en el lugar de inyección
- reacciones alérgicas tales como erupción cutánea («frecuente» en niños de entre 6 meses y <2 años de edad) o picor
- sensación de debilidad o falta de energía/somnolencia
- disminución del apetito («muy frecuente» en niños de entre 6 meses y <2 años de edad)
- mareo
- sudoración excesiva
- sudoración nocturna
Efectos adversos raros:pueden afectar hasta 1 de cada 1000 personas
- parálisis temporal de un lado de la cara
- reacciones alérgicas tales como urticaria o hinchazón de la cara
Efectos adversos muy raros:pueden afectar hasta 1 de cada 10 000 personas
- inflamación del músculo cardiaco (miocarditis) o inflamación del revestimiento externo del corazón (pericarditis) que puede dar lugar a dificultad para respirar, palpitaciones o dolor torácico
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- reacción alérgica grave
- hinchazón extensa en la extremidad en la que se ha administrado la vacuna
- hinchazón de la cara (puede ocurrir hinchazón de la cara en pacientes que hayan recibido inyecciones de relleno dérmico)
- una reacción cutánea que causa puntos rojos o manchas en la piel, que pueden parecer una diana o un «ojo de buey» con un centro de color rojo oscuro rodeado de anillos rojos más pálidos (eritema multiforme)
- sensación anormal en la piel, como cosquilleo u hormigueo (parestesia)
- disminución de la sensibilidad, especialmente en la piel (hipoestesia)
- hemorragia menstrual abundante (la mayoría de los casos no parecen ser graves y son de carácter temporal)
Comunicación de efectos adversos
Si su hijo experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V e incluir el número de lote si se dispone de él. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Comirnaty
Mantener este medicamento fuera de la vista y del alcance de los niños.
La siguiente información sobre conservación, caducidad y uso y manipulación está destinada a profesionales sanitarios.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en congelador a entre –90 °C y –60 °C.
Conservar en el embalaje original para protegerlo de la luz.
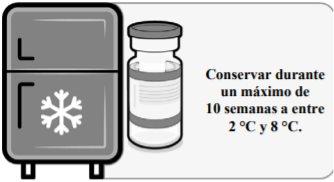
La vacuna se recibe congelada a entre –90 °C y –60 °C. La vacuna congelada se puede conservar a entre –90 °C y –60 °C o a entre 2 °C y 8 °C tras su recepción.
Si se conservan congelados a entre –90 °C y –60 °C, los envases de 10 viales de la vacuna se pueden descongelar a entre 2 °C y 8 °C durante 2 horas o se pueden descongelar viales individuales a temperatura ambiente (hasta 30 °C) durante 30 minutos.
Una vez extraído del congelador, el vial sin abrir puede conservarse y transportarse refrigerado a entre 2 °C y 8 °C durante un máximo de 10 semanas; no superar la fecha de caducidad impresa (CAD). El embalaje exterior se debe marcar con la nueva fecha de eliminación a entre 2 °C y 8 °C. Una vez descongelada, la vacuna no se puede volver a congelar.
Antes de su uso, los viales sin abrir se pueden conservar durante un máximo de 12 horas a temperaturas de entre 8 °C y 30 °C.
Los viales descongelados se pueden manipular en condiciones de luz ambiental.
Tras la dilución, conservar la vacuna a entre 2 °C y 30 °C y usarla en un plazo de 12 horas, que incluye un tiempo de transporte de hasta 6 horas. Desechar la vacuna no utilizada.
No utilice esta vacuna si observa partículas visibles en la dilución o un cambio de color.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Comirnaty
- El principio activo es una vacuna de ARNm frente a COVID-19 llamada tozinamerán. Tras la dilución, el vial contiene 10 dosis de 0,2 ml con 3 microgramos de tozinamerán cada una.
- Los demás componentes son:
- ((4-hidroxibutil)azanodiil)bis(hexano-6,1-diil)bis(2-hexildecanoato) (ALC-0315)
- 2-[(polietilenglicol)-2000]-N,N-ditetradecilacetamida (ALC-0159)
- 1,2-diestearoil-sn-glicero-3-fosfocolina (DSPC)
- colesterol
- trometamol
- hidrocloruro de trometamol
- sacarosa
- agua para preparaciones inyectables
Aspecto del producto y contenido del envase
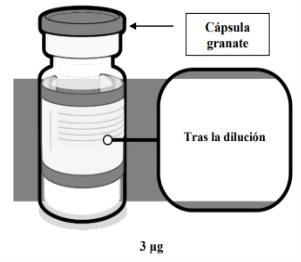
La vacuna es una dispersión (pH: 6,9-7,9) de color entre blanco y blanquecino que se presenta en un vial multidosis de 10 dosis, transparente (vidrio de tipo I), de 2 ml, con un tapón de goma y con una cápsula de cierre de plástico flip-offde color granate con un precinto de aluminio.
Tamaños del envase: 10 viales
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz
Alemania
Teléfono: +49 6131 9084-0
Fax: +49 6131 9084-2121
Responsables de la fabricación
BioNTech Manufacturing GmbH
Kupferbergterrasse 17-19
55116 Mainz
Alemania
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer S.A./N.V. Tél/Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel. +370 52 51 4000 |
| Magyarország Pfizer Kft Tel: +36 1 488 3700 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +35621 344610 |
Danmark Pfizer ApS Tlf: +45 44 201 100 | Norge Pfizer AS Tlf: +47 67 526 100 |
Deutschland BioNTech Manufacturing GmbH Tel: +49 6131 90840 | Nederland Pfizer BV Tel: +31 (0)10 406 43 01 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H Tel: +43 (0)1 521 15-0 |
Ελáδα Pfizer Ελλáς A.E. Τηλ.: +30 210 6785 800 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
España Pfizer, S.L. Tel:+ 34914909900 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél +33 1 58 07 34 40 | România Pfizer Romania S.R.L Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel.: +386 (0) 1 52 11 400 |
Ireland Pfizer Healthcare Ireland Tel: 1800 633 363 (toll free) +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: +421 2 3355 5500 |
Ísland Icepharma hf Simi: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Κúπρος Pfizer Ελλáς Α.Ε. (Cyprus Branch) Tηλ: +357 22 817690 | United Kingdom (Northern Ireland) Pfizer Limited Tel: +44 (0) 1304 616161 |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel.: +371 670 35 775 |
Fecha de la última revisión de este prospecto:
Escanee el código con un dispositivo móvil para obtener el prospecto en diferentes idiomas.

URL: www.comirnatyglobal.com
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
En la página web de la Agencia Europea de Medicamentos puede encontrarse este prospecto en todas las lenguas de la Unión Europea/Espacio Económico Europeo.
Esta información está destinada únicamente a profesionales sanitarios:
Administre Comirnaty por vía intramuscular tras la dilución en una pauta de 3 dosis (0,2 ml cada una); la segunda dosis de la misma vacuna se administra 3 semanas después de la primera dosis seguida de una tercera dosis al menos 8 semanas después de la segunda dosis para completar la pauta de vacunación.
Trazabilidad
Con objeto de mejorar la trazabilidad de los medicamentos biológicos, el nombre y el número de lote del medicamento administrado deben estar claramente registrados.
Instrucciones para la manipulación
Comirnaty 3 microgramos/dosis debe ser preparado por un profesional sanitario empleando una técnica aséptica para garantizar la esterilidad de la dispersión preparada.
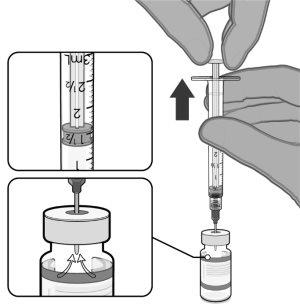
VERIFICACIÓN DEL VIAL DE COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSIÓN INYECTABLE (LACTANTES Y NIÑOS DE ENTRE 6 MESES Y 4 AÑOS DE EDAD) | |
|
|
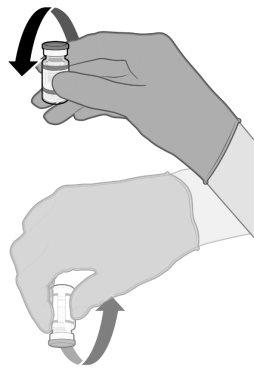
MANEJO ANTES DEL USO DE COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSIÓN INYECTABLE (LACTANTES Y NIÑOS DE ENTRE 6 MESES Y 4 AÑOS DE EDAD) | |
|
Asegúrese de que los viales están completamente descongelados antes de usarlos.
|
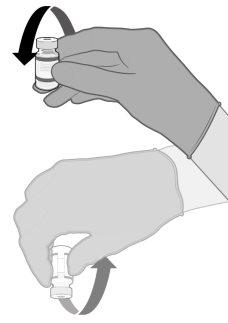
MEZCLA ANTES DE LA DILUCIÓN DE COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSIÓN INYECTABLE (LACTANTES Y NIÑOS DE ENTRE 6 MESES Y 4 AÑOS DE EDAD) | |
Suavemente 10 veces |
|
DILUCIÓN DE COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSIÓN INYECTABLE (LACTANTES Y NIÑOS DE ENTRE 6 MESES Y 4 AÑOS DE EDAD) | |
2,2 ml de una solución inyectable de cloruro sódico a 9 mg/ml (0,9 %). |
|
Tire del émbolo hasta 2,2 ml para extraer aire del vial. |
|
Suavemente 10 veces |
|
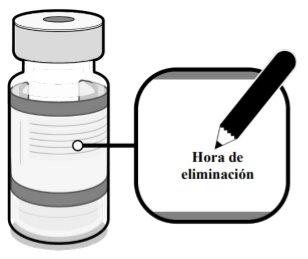
Anote la fecha y la hora apropiadas. Se debe usar en las 12 horas siguientes a la dilución. |
|
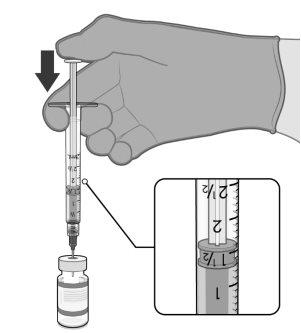
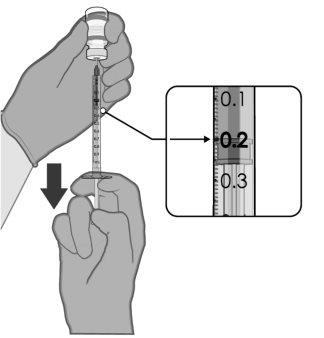
PREPARACIÓN DE DOSIS INDIVIDUALES DE 0,2 ml DE COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSIÓN INYECTABLE (LACTANTES Y NIÑOS DE ENTRE 6 MESES Y4 AÑOS DE EDAD) | |
0,2 ml de vacuna diluida |
Para extraer 10 dosis de un mismo vial se deben utilizar jeringas y/o agujas con un volumen muerto bajo. La combinación de jeringa y aguja con un volumen muerto bajo debe tener un volumen muerto de 35 microlitros como máximo. Si se utilizan jeringas y agujas convencionales, puede no haber el volumen suficiente para extraer 10 dosis de un mismo vial.
|
Eliminación
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSION INYECTABLEForma farmacéutica: INYECTABLE, 0.1 mg/mlPrincipio activo: covid-19, RNA-based vaccineFabricante: Biontech Manufacturing GmbhRequiere recetaForma farmacéutica: INYECTABLE, 0,3 microgramos/0,3 mlPrincipio activo: covid-19, RNA-based vaccineFabricante: Biontech Manufacturing GmbhRequiere recetaForma farmacéutica: INYECTABLE, 10 microgramosPrincipio activo: covid-19, RNA-based vaccineFabricante: Biontech Manufacturing GmbhRequiere receta
Médicos online para COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de COMIRNATY 3 MICROGRAMOS/DOSIS CONCENTRADO PARA DISPERSION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes