
ACETILCISTEINA KERN PHARMA 100 MG/ML SOLUCION INYECTABLE EFG

Cómo usar ACETILCISTEINA KERN PHARMA 100 MG/ML SOLUCION INYECTABLE EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Acetilcisteína Kern Pharma 100 mg/ml solución inyectable EFG
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Acetilcisteína Kern Pharma y para qué se utiliza
- Qué necesita saber antes de empezar a usar Acetilcisteína Kern Pharma
- Cómo usar Acetilcisteína Kern Pharma
- Posibles efectos adversos
- Conservación de Acetilcisteína Kern Pharma
- Contenido del envase e información adicional
1. Qué es Acetilcisteína Kern Pharma y para qué se utiliza
Acetilcisteína Kern Pharma contiene el principio activo acetilcisteína, que pertenece a un grupo de medicamentos denominados mucolíticos que disminuyen la viscosidad del moco, fluidificándolo y facilitando su eliminación de las vías respiratorias.
Acetilcisteína Kern Pharma está indicado para facilitar la eliminación del exceso de mocos y flemas en adultos y niños a partir de 2 años, en los procesos respiratorios en presencia de hipersecreción bronquial como: Bronquitis aguda y crónica, enfermedad pulmonar obstructiva crónica (EPOC), enfisema, complicaciones pulmonares de la fibrosis quística, facilitación de maniobras en anestesia en broncoscopias, broncografías y broncoaspiración, bronquiectasias, complicaciones obstructivas e infecciosas por traqueotomía y broncopulmonares por intervención quirúrgica.
2. Qué necesita saber antes de empezar a usar Acetilcisteína Kern Pharma
No use Acetilcisteína Kern Pharma:
- Si es alérgico al principio activo o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- No administrar en niños menores de 2 años.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar acetilcisteína.
Si es usted asmático o padece una enfermedad respiratoria grave, deberá consultar con el médico antes de tomar este medicamento ya que puede provocar dificultades respiratorias (broncoespasmo).
El posible olor azufrado (a huevos podridos) del medicamento es propio del principio activo, pero no indica que se hayan alterado sus características.
Durante los primeros días de tratamiento podrá observar un aumento de mocos y flemas, que irá disminuyendo a lo largo del tratamiento. Si ve que no es capaz de expectorar de forma efectiva, debe llevarse a cabo un drenaje postural y broncoaspiración.
La administración por vía intravenosa se llevará a cabo bajo una estricta supervisión médica. Es más probable que aparezcan reacciones adversas tras la perfusión intravenosa si el fármaco se administra de una forma demasiado rápida o en una cantidad excesiva. Por lo tanto, se recomienda seguir estrictamente las indicaciones que aparecen en la sección 3. Cómo usar Acetilcisteína Kern Pharma.
La acetilcisteína no es compatible con goma y determinados metales, especialmente hierro, níquel y cobre. Se debe evitar el contacto con materiales que los contengan.
Se debe administrar con precaución en el tratamiento de larga duración en pacientes con intolerancia histamínica.
Niños y adolescentes
En niños y adolescentes son válidas las mismas precauciones y advertencias expuestas.
Está contraindicado en niños menores de 2 años.
Uso de Acetilcisteína Kern Pharma con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
En caso de que necesite tratamiento simultáneo con nitroglicerina, deberá controlarse la aparición de hipotensión (bajada de la presión arterial), que puede ser grave, pudiendo aparecer dolor de cabeza.
El uso simultáneo de carbamazepina, un fármaco usado para combatir los ataques de epilepsia, puede aumentar el riesgo de ataques.
No administrar conjuntamente con medicamentos antitusivos (para la tos) o con aquéllos que disminuyen las secreciones bronquiales (como los antihistamínicos y los anticolinérgicos), ya que podría conducir a una acumulación de secreciones bronquiales.
Se recomienda la administración por separado de antibióticos.
No se recomienda la disolución de acetilcisteína con otros medicamentos.
Uso de Acetilcisteína Kern Pharma con alimentos y bebidas
La toma de alimentos y bebidas no afecta a la eficacia de este medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
La acetilcisteína atraviesa la placenta. Por tanto, no se recomienda el uso de acetilcisteína durante el embarazo.
Se desconoce si acetilcisteína y sus metabolitos se excretan en leche materna. Se debe evitar su uso durante la lactancia.
No se dispone de datos acerca del efecto de acetilcisteína sobre la fertilidad en el ser humano. Los estudios en animales no indican efectos perjudiciales en relación con la fertilidad humana a las dosis recomendadas.
Conducción y uso de máquinas
No existe evidencia de efectos sobre la capacidad para conducir y utilizar máquinas.
Acetilcisteína Kern Pharma contiene sodio
Este medicamento contiene 41,2 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada ampolla de 3 ml. Esto equivale al 2,06% de la ingesta diaria máxima de sodio recomendada para un adulto.
Interferencias con pruebas analíticas
La acetilcisteína puede interferir con el método de valoración colorimétrica para la determinación de salicilatos.
La acetilcisteína puede interferir con el ensayo de cetonas en orina.
3. Cómo usar Acetilcisteína Kern Pharma
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es:
Administración local:
Vía inhalatoria por nebulizador
Adultos y niños a partir de 12 años: una ampolla de 300 mg una o dos veces al día durante 5 a 10 días.
Niños entre 2 y 12 años: hasta una ampolla de 300 mg una o dos veces al día durante 5 a 10 días en los niños que cooperen.
Vía endotraqueobronquial
Adultos y niños a partir de 12 años: una ampolla de 300 mg (60 gotas) una o dos veces al día durante 5 a 10 días.
Niños entre 2 y 12 años: hasta una ampolla de 300 mg (60 gotas) una o dos veces al día durante 5 a 10 días.
Administración parenteral:
Este medicamento puede administrarse en las afecciones bronquiales cuando sea imposible o dificultoso el tratamiento por vía local o cuando el médico prefiera la vía sistémica (falta de cooperación por parte del paciente, reposo obligado en cama, respiración en circuito cerrado, etc.).
Vía intramuscular
Adultos y niños a partir de 12 años:una ampolla de 300 mg una o dos veces al día administrada mediante inyección profunda.
Niños entre 2 y 12 años:150 mg (media ampolla de 3 ml) una o dos veces al día administrada mediante inyección profunda.
Vía intravenosa
La administración de acetilcisteína por vía intravenosa se realiza bajo estricta supervisión médica.
El medicamento debe administrarse mediante perfusión lenta en solución salina o solución de glucosa al 5%.
Adultos y niños a partir de 12 años: una ampolla de 300 mg una o dos veces al día.
Niños entre 2 y 12 años: 150 mg (media ampolla de 3 ml) una o dos veces al día.
Apertura de la ampolla:
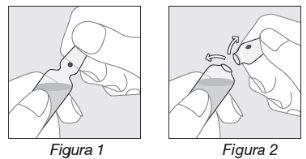
- Sostener la ampolla según se indica en la Figura 1;
- Colocar el pulgar en el punto de color y ejercer presión hacia atrás según se indica en la Figura 2.
Duración del tratamiento
La duración del tratamiento debe ser establecida de acuerdo a la evolución clínica. La duración media es de 5-10 días. La elevada tolerabilidad general y local de acetilcisteína permite tratamientos prolongados en ciertos casos.
Si usa más Acetilcisteína Kern Pharma de la que debe
Si usted ha utilizado más acetilcisteína de la que debe puede notar síntomas similares aunque más intensos que los descritos en la sección 4. Posibles efectos adversos. En caso de sobredosis o administración masiva accidental, consulte inmediatamente a su médico o farmacéutico o bien llame al Servicio de Información Toxicológica, teléfono 91.562.04.20 indicando el medicamento y la cantidad utilizada.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se pueden producir los siguientes efectos adversos, aunque su frecuencia no puede establecerse a partir de la información disponible:
Uso local:
Reacciones de alergia (hipersensibilidad), constricción de los bronquios y dificultad para respirar (broncoespasmo), secreción nasal aumentada (rinorrea), aftas bucales (estomatitis), vómitos, náuseas, urticaria, erupción o picor.
Uso parenteral:
Reacciones de alergia (hipersensibilidad) de diverso grado pudiendo llegar a shock anafiláctico, aumento de la frecuencia de los latidos del corazón (taquicardia), constricción de los bronquios y dificultad para respirar (broncoespasmo, disnea), vómitos, náuseas, hinchazón facial (angioedema), urticaria, rubor, erupción, picor, edema facial, disminución de la presión sanguínea, disminución de la coagulación sanguínea (aumento del tiempo de protrombina, disminución de la agregación plaquetaria).
En casos muy raros, aparición de reacciones cutáneas graves (síndrome de Stevens-Johnson y síndrome de Lyell) aunque en la mayoría de los casos, se pudo identificar también al menos otro fármaco sospechoso de desencadenar el síndrome.
En caso de producirse cualquier alteración en la piel o membranas mucosas, debe interrumpirse inmediatamente la administración de acetilcisteína. El médico especialista determinará el tratamiento a seguir.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Acetilcisteína Kern Pharma
Mantener este medicamento fuera de la vista y del alcance de los niños.
No requiere condiciones especiales de conservación.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de la abreviatura CAD. La fecha de caducidad es el último día del mes que se indica.
Administración local
Se aconseja abrir la ampolla en el momento de su empleo. Las ampollas abiertas sólo podrán utilizarse para uso local, debiéndose conservar en nevera durante un máximo de 24 horas.
Administración parenteral
Una vez abierto, utilizar inmediatamente. Si no se utiliza inmediatamente, los tiempos y condiciones de conservación en uso son responsabilidad del usuario.
La solución, una vez diluida para su uso (en solución de glucosa al 5% o en solución de cloruro de sodio al 0,9%) se mantiene estable durante un período de 24 horas a 25ºC.
Desechar después de usar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesite en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Acetilcisteína Kern Pharma
El principio activo es acetilcisteína. Cada ampolla de 3 ml contiene 300 mg de acetilcisteína.
Los demás componentes son: edetato de disodio, hidróxido de sodio (para ajuste del pH) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Aspecto: ampollas de vidrio topacio con punto de rotura, conteniendo 3 ml de solución transparente e incolora.
Cada envase contiene 5 ampollas.
Titular de la autorización de comercialización y responsable de la fabricación
Kern Pharma, S.L.
Venus, 72 – Pol. Ind. Colón II
08228 Terrassa - Barcelona
España
Fecha de la última revisión de este prospecto:Diciembre 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ACETILCISTEINA KERN PHARMA 100 MG/ML SOLUCION INYECTABLE EFGForma farmacéutica: COMPRIMIDO EFERVESCENTE, 600 MGPrincipio activo: AcetilcisteinaFabricante: Kern Pharma S.L.No requiere recetaForma farmacéutica: COMPRIMIDO EFERVESCENTE, 600 mgPrincipio activo: AcetilcisteinaFabricante: Laboratorios Alter S.A.Requiere recetaForma farmacéutica: COMPRIMIDO EFERVESCENTE, 200 mgPrincipio activo: AcetilcisteinaFabricante: Aurovitas Spain, S.A.U.Requiere receta
Médicos online para ACETILCISTEINA KERN PHARMA 100 MG/ML SOLUCION INYECTABLE EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ACETILCISTEINA KERN PHARMA 100 MG/ML SOLUCION INYECTABLE EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















