
Darunavir Sinoptis

Consulta con un médico sobre la receta médica de Darunavir Sinoptis

Cómo usar Darunavir Sinoptis
Hoja de instrucciones del paquete: información para el usuario
Darunavir Synoptis, 75 mg, tabletas recubiertas
Darunavir Synoptis, 150 mg, tabletas recubiertas
Darunavir Synoptis, 300 mg, tabletas recubiertas
Darunavir Synoptis, 600 mg, tabletas recubiertas
Darunavir
Es importante leer detenidamente el contenido de esta hoja de instrucciones antes de tomar el medicamento, ya que contiene información importante para el paciente.
- Debe conservar esta hoja de instrucciones para poder volver a leerla si es necesario.
- En caso de dudas, debe consultar a su médico, farmacéutico o enfermera.
- Este medicamento ha sido recetado específicamente para usted. No debe dárselo a otros. El medicamento puede ser perjudicial para otra persona, incluso si los síntomas de su enfermedad son los mismos.
- Si el paciente experimenta algún efecto adverso, incluidos los efectos adversos no mencionados en esta hoja de instrucciones, debe informar a su médico, farmacéutico o enfermera. Véase el punto 4.
Índice de la hoja de instrucciones
- 1. Qué es Darunavir Synoptis y para qué se utiliza
- 2. Información importante antes de tomar Darunavir Synoptis
- 3. Cómo tomar Darunavir Synoptis
- 4. Posibles efectos adversos
- 5. Cómo conservar Darunavir Synoptis
- 6. Contenido del paquete y otra información
1. Qué es Darunavir Synoptis y para qué se utiliza
Qué es Darunavir Synoptis?
Darunavir Synoptis contiene la sustancia activa darunavir. Darunavir Synoptis es un medicamento antirretroviral utilizado para tratar infecciones por el virus de la inmunodeficiencia humana (VIH). El medicamento pertenece a la clase de inhibidores de la proteasa. El efecto de Darunavir Synoptis consiste en reducir la cantidad de virus VIH en el organismo. Esto ayuda al sistema inmunológico y reduce el riesgo de contraer enfermedades relacionadas con la infección por el virus VIH.
Para qué se utiliza?
Darunavir Synoptis se utiliza para tratar a adultos y niños mayores de 3 años y con un peso corporal superior a 15 kg infectados con el virus VIH que han sido tratados previamente con otros medicamentos antirretrovirales. Darunavir Synoptis debe tomarse en combinación con una dosis baja de ritonavir y otros medicamentos contra el virus VIH. El médico determinará la mejor combinación de medicamentos para el paciente.
2. Información importante antes de tomar Darunavir Synoptis
Cuándo no tomar Darunavir Synoptis
- si el paciente es alérgico a darunavir o a cualquier otro componente de este medicamento (enumerados en el punto 6) o a ritonavir,
- si el paciente tiene trastornos hepáticos graves. En caso de dudas sobre problemas hepáticos y su naturaleza, debe consultar a un médico. Es posible que se requieran algunas pruebas adicionales.
No tomar Darunavir Synoptis con los siguientes medicamentos
En caso de tomar alguno de los medicamentos enumerados a continuación, debe consultar a un médico para cambiar el tratamiento.
| Nombre del medicamento | Uso del medicamento |
| Awanafil | tratamiento de la disfunción eréctil |
| Astemizol o terfenadina | tratamiento de los síntomas alérgicos |
| Triazolam y midazolam oral | efecto sedante y/o ansiolítico |
| Cizaprida | tratamiento de algunos trastornos gastrointestinales |
| Colchicina (en trastornos renales y/o hepáticos) | tratamiento de la gota o la fiebre mediterránea familiar |
| Lurasidona, pimozida, quetiapina o sertindol | tratamiento de trastornos psiquiátricos |
| Alcaloides del cornezuelo, como ergotamina, dihidroergotamina, ergometrina y metilergonovina | tratamiento de migrañas |
| Amiodarona, bepridil, dronedarona, ivabradina, quinidina, ranolazina | tratamiento de algunas enfermedades cardíacas, como trastornos del ritmo |
| Lowastatina, simvastatina y limitapida | tratamiento para reducir los niveles de colesterol en la sangre |
| Rifampicina | tratamiento de algunas infecciones, como la tuberculosis |
| Medicamento combinado lopinavir/ritonavir | medicamento contra el virus VIH, perteneciente al mismo grupo que Darunavir Synoptis |
| Elbasvir/grazoprevir | tratamiento de la infección por el virus de la hepatitis C |
| Alfuzosina | tratamiento de la hiperplasia prostática |
| Sildenafil | tratamiento de la hipertensión pulmonar |
| Dabigatrán, ticagrelor | prevención de la agregación plaquetaria en pacientes con infarto de miocardio previo |
| Naloxegol | tratamiento de la constipación inducida por opioides |
| Dapoxetina | tratamiento de la eyaculación precoz |
| Domperidona | tratamiento de las náuseas y los vómitos |
During the therapy with Darunavir Synoptis, you must not take products containing Hypericum perforatum(St. John's Wort).
Precauciones y advertencias
Before starting treatment with Darunavir Synoptis, you must discuss it with your doctor, pharmacist, or nurse. Darunavir Synoptis will not cure your HIV infection. You can still transmit HIV to others when taking this medication, despite effective antiretroviral therapy reducing this risk. You should discuss with your doctor the precautions needed to avoid infecting others. People taking Darunavir Synoptis are still at risk of developing infections or other diseases related to the presence of HIV, so you should maintain regular contact with your doctor. A skin rash may occur in patients taking Darunavir Synoptis. Rarely, it can be severe or life-threatening. If a rash occurs, you should contact your doctor. Rash (usually mild or moderate) may occur more frequently in patients taking Darunavir Synoptis and raltegravir than in patients taking either drug separately.
When to inform your doctor about your health status BEFORE and DURING treatment
After reviewing the following points, you should inform your doctor if any of them apply to you.
- You should inform your doctor if you have had liver diseasein the past, including hepatitis B or C infection. Your doctor will assess how severe the disease was before deciding whether you can take Darunavir Synoptis.
- You should inform your doctor if you have diabetes. Darunavir Synoptis may increase your blood sugar levels.
- You should immediately inform your doctor if you notice infection symptoms(e.g., swollen lymph nodes, fever). In some patients with advanced HIV infection and a history of opportunistic infections, symptoms of inflammation related to previous infections may appear immediately after starting antiretroviral therapy. It is believed that the appearance of such symptoms is related to the strengthening of the immune system, allowing the body to fight existing asymptomatic infections.
- In addition to opportunistic infections, autoimmune diseases (diseases that occur when the immune system attacks healthy tissues) may also occur after starting antiretroviral therapy. Autoimmune diseases can occur months after starting treatment. If you notice symptoms of infection or other symptoms such as muscle weakness, weakness starting from the hands and feet and progressing towards the torso, heart palpitations, tremors, or hyperactivity, you should contact your doctor as soon as possible to start the necessary treatment.
- You should inform your doctor if you have hemophilia. Darunavir Synoptis may increase the risk of bleeding.
- You should inform your doctor about sulfonamide allergy(used, for example, in the treatment of certain infections).
- You should inform your doctor about musculoskeletal disorders. In some patients taking combined antiretroviral therapy, a bone disease called osteonecrosis (bone death due to insufficient blood supply) may develop. Risk factors for developing this disease include: long-term use of combined antiretroviral therapy, use of corticosteroids, alcohol consumption, severe immunosuppression, increased body mass index, and others. Symptoms of osteonecrosis include: joint pain and stiffness (especially in the hips, knees, or shoulders) and difficulty moving. You should inform your doctor if you experience any of these symptoms.
Older adults
Darunavir Synoptis has only been used in a small number of patients aged 65 or older. If you are in this age group, you should consult your doctor to discuss the possibility of taking this medication.
Children
Darunavir Synoptis should not be used in children under 3 years of age or weighing less than 15 kilograms.
Darunavir Synoptis and other medications
You should tell your doctor or pharmacist about all the medications you are currently taking or have recently taken. Some medications should not be takenwith Darunavir Synoptis. Their list is provided in the section "Do not take Darunavir Synoptis with the following medications". In most cases, Darunavir Synoptis can be taken with HIV medications from another group [e.g., NRTI (nucleoside reverse transcriptase inhibitors), NNRTI (non-nucleoside reverse transcriptase inhibitors), CCR5 antagonists, and fusion inhibitors]. However, studies have not been conducted on the simultaneous use of Darunavir Synoptis and ritonavir with all PI (protease inhibitors), and it should not be used in combination with other protease inhibitors. In some cases, it may be necessary to change the dosage of other medications. Therefore, in each case, you should inform your doctor about the use of other HIV medications and strictly follow the doctor's recommendations regarding the simultaneous use of other medications. The effectiveness of Darunavir Synoptis may be reduced when taken with one of the following products. You should inform your doctor about taking:
- phenobarbital, phenytoin(antiepileptic medications);
- dexamethasone(corticosteroid);
- efavirenz(HIV-1 infection);
- boceprevir(hepatitis C virus infection);
- rifapentine, rifabutin(antituberculosis medications);
- saquinavir(HIV-1 infection).
Taking Darunavir Synoptis may affect the effectiveness of other medications. You should inform your doctor about taking:
- amlodipine, diltiazem, disopyramide, carvedilol, felodipine, flecainide, lidocaine, metoprolol, mexiletine, nifedipine, nicardipine, propafenone, timolol, verapamil(cardiac medications), due to the potential for increased therapeutic effects and adverse reactions of these medications;
- apixaban, edoxaban, rivaroxaban, warfarin(anticoagulant medication), due to the potential for increased therapeutic effects and adverse reactions of these medications; blood tests may be necessary;
- hormonal contraceptives and hormone replacement therapy; Darunavir Synoptis may reduce their effectiveness; to avoid pregnancy, it is recommended to use other non-hormonal methods of birth control;
- ethinyl estradiol with drospirenone. Darunavir Synoptis may increase the risk of hyperkalemia associated with drospirenone;
- atorvastatin, pravastatin, rosuvastatin(cholesterol-lowering medications); there is an increased risk of muscle damage. Your doctor will assess which cholesterol-lowering medication is suitable for you in this situation;
- clarithromycin(antibiotic);
- cyclosporine, everolimus, tacrolimus, sirolimus(immunosuppressants), due to the potential for increased therapeutic effects and adverse reactions of these medications - your doctor may recommend additional tests;
- corticosteroids, including betamethasone, budesonide, fluticasone, mometasone, prednisone, triamcinolone. These medications are used to treat allergies, asthma, inflammatory bowel diseases, eye inflammation, joint and muscle inflammation, and other inflammatory conditions. If there are no alternative medications, the use of these medications is possible only after the doctor's assessment and under the close monitoring of corticosteroid adverse reactions by the attending physician;
- buprenorphine/naloxone(medications used to treat opioid addiction);
- salmeterol(medication used to treat asthma);
- artemether/lumefantrine(combined medication used to treat malaria);
- dasatinib, everolimus, irinotecan, nilotinib, vinblastine, vincristine(medications used to treat cancer);
- sildenafil, tadalafil, vardenafil(medications used to treat erectile dysfunction or pulmonary arterial hypertension);
- glecaprevir/pibrentasvir, simprevir(used to treat hepatitis C virus infection);
Fentanyl, oxycodone, tramadol(pain medications);
- fesoterodine, solifenacin(used to treat urological disorders).
This is not a complete list of medications. You should inform your doctor about allmedications you are taking.
Darunavir Synoptis with food and drink
See section 3 "How to take Darunavir Synoptis".
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medication. Women who are pregnant or breastfeeding should not take Darunavir Synoptis with ritonavir without special recommendation from their doctor. Women who are pregnant or breastfeeding should not take Darunavir Synoptis with cobicistat. HIV-infected women should not breastfeed their babies because they can transmit the virus to their babies through breast milk, and the effects of the medication on the baby are not known.
Driving and using machines
You should not drive or operate machinery if taking Darunavir Synoptis causes dizziness.
Darunavir Synoptis contains lactose
This medication contains lactose. If you have been diagnosed with an intolerance to some sugars, you should contact your doctor before taking this medication.
Darunavir Synoptis, 75 mg, film-coated tablets contain propylene glycol (E1520).
This medication contains 10.42 mg of propylene glycol (E1520) per film-coated tablet. If the child to be treated is less than 4 weeks old, you should discuss this with your doctor or pharmacist before giving this medication, especially if the child is taking other medications containing propylene glycol or alcohol.
Darunavir Synoptis, 150 mg, film-coated tablets contain propylene glycol (E1520).
This medication contains 20.84 mg of propylene glycol (E1520) per film-coated tablet. If the child to be treated is less than 4 weeks old, you should discuss this with your doctor or pharmacist before giving this medication, especially if the child is taking other medications containing propylene glycol or alcohol.
Darunavir Synoptis, 300 mg, film-coated tablets contain propylene glycol (E1520).
This medication contains 41.66 mg of propylene glycol (E1520) per film-coated tablet. If the child to be treated is less than 4 weeks old, you should discuss this with your doctor or pharmacist before giving this medication, especially if the child is taking other medications containing propylene glycol or alcohol. Darunavir Synoptis, 300 mg, film-coated tablets contain orange yellow S (E 110), which may cause allergic reactions.
Darunavir Synoptis, 600 mg, film-coated tablets contain propylene glycol (E1520).
This medication contains 83.33 mg of propylene glycol (E1520) per film-coated tablet. If the child is less than 4 weeks old, you should discuss this with your doctor or pharmacist before giving this medication, especially if the child is taking other medications containing propylene glycol or alcohol. Darunavir Synoptis, 600 mg, film-coated tablets contain orange yellow S (E 110), which may cause allergic reactions.
3. How to take Darunavir Synoptis
This medication should always be taken as described in the patient information leaflet or as directed by your doctor, pharmacist, or nurse. If you have any doubts, you should consult your doctor, pharmacist, or nurse. If you notice significant improvement, you should not stop taking Darunavir Synoptis with ritonavir without consulting your doctor. After starting treatment, you should not change the dose, form of the medication, or stop treatment without your doctor's recommendation.
Dosing in children over 3 years old, weighing at least 15 kg, who have not taken antiretroviral medications before (the attending physician will determine this)
The doctor will determine the appropriate dose to be taken once a day based on the child's weight (see the table below). This dose should not exceed the recommended dose for adults, which is 800 mg of Darunavir Synoptis and 100 mg of ritonavir once a day. The doctor will inform you how much darunavir and ritonavir (in capsules, tablets, or solution) the child should take. There are tablets of different strengths, and the doctor may recommend using a combination of tablets to achieve the desired dosing regimen. Darunavir may also be available as an oral suspension. The doctor will determine whether darunavir tablets or oral suspension are suitable for the child.
| Weight | Single dose of darunavir | Single dose of ritonavir |
| from 15 to 30 kilograms | 600 milligrams | 100 milligrams |
| from 30 to 40 kilograms | 675 milligrams | 100 milligrams |
| from 40 kilograms | 800 milligrams | 100 milligrams |
Dosing in children over 3 years old, weighing at least 15 kg, who have taken antiretroviral medications before (the attending physician will determine this)
The doctor will determine the appropriate dose based on the child's weight (see the table below). The doctor will assess whether the child should take the medication once or twice a day. This dose should not exceed the recommended dose for adults, which is 600 mg of darunavir and 100 mg of ritonavir twice a day, or 800 mg of Darunavir Synoptis and 100 mg of ritonavir once a day. The doctor will inform you how much darunavir and ritonavir (in capsules, tablets, or solution) the child should take. There are tablets of different strengths, and the doctor may recommend using a combination of tablets to achieve the desired dosing regimen. Darunavir is also available as an oral suspension. The doctor will determine whether darunavir tablets or oral suspension are suitable for the child. Twice-daily dosing: Once-daily dosing: Instructions for taking Darunavir Synoptis in children:
- The child must take Darunavir Synoptis in combination with ritonavir. Darunavir Synoptis does not work properly without ritonavir.
- The child must take the appropriate dose of Darunavir Synoptis and ritonavir twice a day or once a day. If Darunavir Synoptis is prescribed twice a day, the child should take one dose in the morning and the second dose in the evening. Each dose should be taken with ritonavir and food.
- The child must take Darunavir Synoptis with food. Darunavir Synoptis does not work properly when taken without food. The type of food is not important.
- The child must swallow the tablets with water or milk.
Dosing in adults who have not taken antiretroviral medications before (the attending physician will determine this)
The patient requires a different dose of Darunavir Synoptis, which cannot be administered using these tablets. Other strengths of Darunavir Synoptis are available.
Dosing in adults who have taken antiretroviral medications before (the attending physician will determine this)
The dose is: 600 mg of darunavir with 100 mg of ritonavir twice a day. OR 800 mg of Darunavir Synoptis (2 tablets of 400 mg or 1 tablet of 800 mg) with 100 mg of ritonavir once a day. To use the 800 mg regimen, only 400 mg or 800 mg Darunavir Synoptis tablets should be used. You should discuss with your doctor which dose is suitable for you.
Opening the child-resistant cap
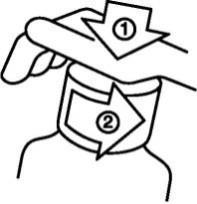
The plastic bottle is equipped with a child-resistant cap. To open it, follow these steps:
- Press the plastic cap while turning it counterclockwise;
- Remove the opened cap.
Taking a higher dose of Darunavir Synoptis than recommended
You should contact your doctor, pharmacist, or nurse immediately.
Missing a dose of Darunavir Synoptis
If a dose is missed within 6 hours, you should take the missed tablets as soon as possible. Each dose should be taken with ritonavir and food. If a dose is missed after 6 hours, you should skip the missed dose and take the next dose at the usual time. Do not take a double dose to make up for the missed dose.
Do not stop taking Darunavir Synoptis without consulting your doctor
Antiretroviral medications can make you feel better, but even if you notice improvement, you should not stop taking Darunavir Synoptis with ritonavir without consulting your doctor. If you have any further doubts about taking this medication, you should consult your doctor, pharmacist, or nurse.
4. Possible side effects
During HIV treatment, weight gain and increased blood lipid and glucose levels may occur. This is partly related to improved health and lifestyle, and in the case of blood lipid levels, sometimes to the use of antiretroviral medications themselves. Your doctor will order tests to monitor these changes. Like all medications, Darunavir Synoptis can cause side effects, although not everyone gets them.
If you experience any of the following side effects, you should inform your doctor.
Liver problems have been reported, which have occasionally been severe. Your doctor will order blood tests before starting Darunavir Synoptis. If you have chronic hepatitis B or C, your doctor should order blood tests more frequently, as there is an increased risk of liver problems. You should discuss with your doctor the symptoms of liver dysfunction. These may include: yellowing of the skin or eyes, dark urine (tea-colored), pale stools, nausea, vomiting, loss of appetite, or pain or discomfort in the area below the ribs on the right side. Skin rash (more common when taking raltegravir), itching. The rash is usually mild to moderate. However, skin rash can also be a symptom of a rare but severe condition. If you notice this symptom, you should discuss it with your doctor. Your doctor will recommend appropriate treatment for the symptoms or decide to stop Darunavir Synoptis. Other serious side effects have included diabetes (often) and pancreatitis (not very often). Very common side effects(may affect more than 1 in 10 people)
- Diarrhea.
Common side effects(may affect up to 1 in 10 people)
- Vomiting, nausea, abdominal pain or bloating, indigestion, bloating;
- Headache, fatigue, dizziness, drowsiness, numbness, tingling, or pain in the hands or feet, weakness, difficulty sleeping.
Uncommon side effects(may affect up to 1 in 100 people)
- Chest pain, changes in the ECG, rapid heart rate;
- Disorders or decreased sensation, numbness, tingling, concentration disorders, memory loss, balance disorders;
- Breathing difficulties, cough, nosebleeds, throat irritation;
- Gastritis or oral inflammation, heartburn, gagging, dry mouth, unpleasant abdominal sensations, constipation, belching;
- Kidney failure, kidney stones, difficulty urinating, frequent or excessive urination, sometimes at night;
- Hives, severe skin and tissue swelling (most often around the lips or eyes), rash, excessive sweating, night sweats, hair loss, acne, scaly skin, nail pigmentation;
- Muscle pain, muscle cramps, or decreased muscle strength, joint pain with or without inflammation;
- Decreased thyroid activity, which can be detected by blood tests;
- Hypertension, sudden flushing of the face;
- Redness or dryness of the eyes;
- Fever, swelling of the lower limbs due to fluid retention, feeling unwell, pain;
- Infection symptoms, herpes;
- Erectile dysfunction, breast enlargement in men;
- Sleep disorders, drowsiness, depression, anxiety, strange dreams, decreased libido.
Rare side effects(may affect up to 1 in 1,000 people)
- DRESS syndrome [severe rash, which may be accompanied by fever, fatigue, facial or lymph node swelling, increased eosinophil count (a type of white blood cell), liver, kidney, or lung symptoms];
- Heart attack, slow heart rate, palpitations;
- Visual disturbances;
- Chills, malaise;
- Feeling disoriented or lost, mood changes, restlessness;
- Fainting, seizures, changes or loss of taste;
- Oral pain, bleeding vomiting, oral inflammation, dry mouth, unpleasant oral sensations;
- Cough;
- Diseases of the skin, dry skin;
- Muscle stiffness or joint pain with or without inflammation;
- Blood cell count and biochemical test abnormalities. Changes can be observed in blood or urine test results. Your doctor will provide more detailed explanations. For example, an abnormality may be an increased count of a certain type of white blood cell.
Side effects typical of HIV medications in the same class as Darunavir Synoptis include:
- Muscle pain, pain, or weakness. In rare cases, these disorders have been severe.
Reporting side effects
If you experience any side effects, including those not mentioned in this leaflet, you should inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: 22 49 21 301, fax: 22 49 21 309, website: https://smz.ezdrowie.gov.pl Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medication.
5. How to store Darunavir Synoptis
The medication should be stored out of sight and reach of children. Do not use this medication after the expiration date stated on the carton after "Expiration Date" and on the bottle after "EXP". The expiration date refers to the last day of the specified month. There are no special precautions for storing Darunavir Synoptis. Medications should not be disposed of in wastewater or household waste. You should ask your pharmacist how to dispose of medications that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Darunavir Synoptis contains
- The active substance of the medication is darunavir. Each film-coated tablet of Darunavir Synoptis, 75 mg, contains 75 mg of darunavir (in the form of darunavir with propylene glycol).
Each film-coated tablet of Darunavir Synoptis, 150 mg, contains 150 mg of darunavir (in the form of darunavir with propylene glycol). Each film-coated tablet of Darunavir Synoptis, 300 mg, contains 300 mg of darunavir (in the form of darunavir with propylene glycol). Each film-coated tablet of Darunavir Synoptis, 600 mg, contains 600 mg of darunavir (in the form of darunavir with propylene glycol).
- Other ingredients are: Darunavir Synoptis, 75 mg, film-coated tablets
- microcelac 100 consisting of: lactose monohydrate, microcrystalline cellulose
- povidone K30
- crosprovidone
- silica colloidal anhydrous
- magnesium stearate Coating (White): polyvinyl alcohol, titanium dioxide (E 171), macrogol 3350, talc
Darunavir Synoptis, 150 mg, film-coated tablets
- microcelac 100 consisting of: lactose monohydrate, microcrystalline cellulose
- povidone K30
- crosprovidone
- silica colloidal anhydrous
- magnesium stearate Coating (White): polyvinyl alcohol, titanium dioxide (E 171), macrogol 3350, talc
Darunavir Synoptis, 300 mg, film-coated tablets
- microcelac 100 consisting of: lactose monohydrate, microcrystalline cellulose
- povidone K30
- crosprovidone
- silica colloidal anhydrous
- magnesium stearate Coating (Orange-1): polyvinyl alcohol, titanium dioxide (E 171), macrogol 3350, talc, orange yellow S (E 110)
Darunavir Synoptis, 600 mg, film-coated tablets
- microcelac 100 consisting of: lactose monohydrate, microcrystalline cellulose
- povidone K30
- crosprovidone
- silica colloidal anhydrous
- magnesium stearate Coating (Orange-1): polyvinyl alcohol, titanium dioxide (E 171), macrogol 3350, talc, orange yellow S (E 110)
What Darunavir Synoptis looks like and contents of the pack
Darunavir Synoptis, 75 mg, film-coated tablets White, capsule-shaped tablets with the imprint "75" on one side, measuring: length 9.4 ± 0.2 mm, width 4.5 ± 0.2 mm, and thickness 3.4 ± 0.3 mm. Darunavir Synoptis, 150 mg, film-coated tablets White, oval tablets with the imprint "150" on one side, measuring: length 13.8 ± 0.2 mm, width 7.0 ± 0.2 mm, and thickness 3.6 ± 0.3 mm. Darunavir Synoptis, 300 mg, film-coated tablets Orange, oval tablets with the imprint "300" on one side, measuring: length 16.1 ± 0.2 mm, width 8.1 ± 0.2 mm, and thickness 5.2 ± 0.3 mm. Darunavir Synoptis, 600 mg, film-coated tablets Orange, oval tablets with the imprint "600" on one side, measuring: length 20.2 ± 0.2 mm, width 10.2 ± 0.2 mm, and thickness 6.8 ± 0.4 mm. Carton containing a bottle with HDPE and a PP cap, with a child-resistant closure and a seal. Pack sizes: Darunavir Synoptis, 75 mg, film-coated tablets One bottle containing 480 tablets Darunavir Synoptis, 150 mg, film-coated tablets One bottle containing 240 tablets Darunavir Synoptis, 300 mg, film-coated tablets One bottle containing 120 tablets Darunavir Synoptis, 600 mg, film-coated tablets One bottle containing 60 tablets
Marketing authorization holder and manufacturer:
Marketing authorization holder
Synoptis Pharma Sp. z o.o. ul. Krakowiaków 65 02-255 Warsaw
Manufacturer:
Pharmathen S.A. Dervenakion 6 15351 Pallini Attiki Greece Pharmathen International S.A. Industrial Park Sapes Rodopi Prefecture, Block No 5 69300 Rodopi Greece Pharmadox Healthcare Ltd. KW20A Kordin Industrial Park Paola, PLA 3000 Malta Hormosan Pharma GmbH Hanauer Landstraße 139-143 60314 Frankfurt am Main Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Denmark DAVARINO Germany Darunavir Hormosan 75 mg/150 mg/300 mg/400 mg/600 mg/800 mg film-coated tablets Spain Darunavir Kern Pharma 75/150/300/600 mg film-coated tablets United Kingdom Darunavir 600mg/800mg film-coated tablets Poland Darunavir Synoptis Greece DAVARINO Cyprus DAVARINO Romania Darunavir Flomi 400 mg/600 mg/800 mg film-coated tablets Austria Darunavir Accord 75mg/150mg/400mg/600mg/800mg film-coated tablets Czech Republic Darunavir Accord Slovenia Darunavir Accord 75mg/150mg/300mg/400mg/600mg/800mg film-coated tablets Netherlands Darunavir Accord 75mg/150mg/300mg/400mg/600mg/800mg film-coated tablets Norway Darunavir Accord 600mg/800mg film-coated tablets Italy Darunavir Accord 600mg/800mg film-coated tablets Ireland Malta Darunavir Accord 600mg/800mg film-coated tablets Darunavir Accord 600mg film-coated tablets Bulgaria Darunavir Accord 800mg film-coated tablets Croatia Darunavir Accord 800mg film-coated tablets Finland Darunavir Accord 400mg/600mg/800mg film-coated tablets Sweden Darunavir Accord 400mg/600mg/800mg film-coated tablets Portugal Darunavir Accord 400mg/600mg/800mg film-coated tablets France Darunavir Accord 75mg/150mg/300mg/400mg/600mg/800mg film-coated tablets
Date of last revision of the leaflet: 04/2020
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- ImportadorHormosan Pharma GmbH Pharmadox Healthcare Ltd. Pharmathen International S.A. Pharmathen S.A.
- Esta información ha sido traducida con IA y es solo orientativa. No constituye asesoramiento médico. Consulta siempre con un médico antes de tomar cualquier medicamento.
- Alternativas a Darunavir Sinoptis
Alternativas a Darunavir Sinoptis en otros países
Las mejores alternativas con el mismo principio activo y efecto terapéutico.
Alternativa a Darunavir Sinoptis en España
Alternativa a Darunavir Sinoptis en Ucrania
Médicos online para Darunavir Sinoptis
Consulta sobre dosis, efectos secundarios, interacciones, contraindicaciones y renovación de la receta de Darunavir Sinoptis – sujeta a valoración médica y normativa local.














