YUFLYMA 40 mg Injectable Solution in a Pre-filled Pen

How to use YUFLYMA 40 mg Injectable Solution in a Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Yuflyma 40 mg Solution for Injection in Pre-filled Pen
adalimumab
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- Your doctor will give you a patient information card, which contains important safety information that you need to know before you start using and during treatment with Yuflyma. Carry this patient information cardwith you during treatment and for 4 months after your last injection of Yuflyma.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Yuflyma and what is it used for
- What you need to know before you use Yuflyma
- How to use Yuflyma
- Possible side effects
- Storage of Yuflyma
- Contents of the pack and other information
- Instructions for use
1. What is Yuflyma and what is it used for
Yuflyma contains the active substance adalimumab, a medicine that works on your immune system (defence).
Yuflyma is used to treat the following inflammatory diseases:
- Rheumatoid arthritis
- Polyarticular juvenile idiopathic arthritis
- Enthesitis-related arthritis
- Ankylosing spondylitis
- Non-radiographic axial spondyloarthritis
- Psoriatic arthritis
- Plaque psoriasis
- Hidradenitis suppurativa
- Crohn's disease
- Ulcerative colitis
- Non-infectious uveitis
The active substance in Yuflyma, adalimumab, is a human monoclonal antibody. Monoclonal antibodies are proteins that target a specific target.
The target of adalimumab is a protein called tumour necrosis factor (TNF alpha), which is involved in the immune system (defence) and is found in high levels in the inflammatory diseases mentioned above. By targeting TNF alpha, Yuflyma reduces the inflammation process in these diseases.
Rheumatoid Arthritis
Rheumatoid arthritis is an inflammatory disease of the joints.
Yuflyma is used to treat moderate to severe rheumatoid arthritis in adults. You may have been given other medicines that modify the disease, such as methotrexate, before. If your response to these medicines is not sufficient, you will be given Yuflyma.
Yuflyma can also be used to treat severe, active, and progressive rheumatoid arthritis without prior treatment with methotrexate.
Yuflyma can reduce the damage to your joints caused by the inflammatory disease and help you move more freely.
Your doctor will decide if Yuflyma should be used with methotrexate or alone.
Polyarticular Juvenile Idiopathic Arthritis
Polyarticular juvenile idiopathic arthritis is an inflammatory disease of the joints.
Yuflyma is used to treat polyarticular juvenile idiopathic arthritis in patients from 2 years of age. You may have been given other medicines that modify the disease, such as methotrexate, before. If your response to these medicines is not sufficient, you will be given Yuflyma.
Your doctor will decide if Yuflyma should be used with methotrexate or alone.
Enthesitis-related Arthritis
Enthesitis-related arthritis is an inflammatory disease of the joints and the sites where tendons attach to the bone.
Yuflyma is used to treat enthesitis-related arthritis in patients from 6 years of age. You may have been given other medicines that modify the disease, such as methotrexate, before. If your response to these medicines is not sufficient, you will be given Yuflyma.
Ankylosing Spondylitis and Non-radiographic Axial Spondyloarthritis
Ankylosing spondylitis and non-radiographic axial spondyloarthritis are inflammatory diseases that affect the spine.
Yuflyma is used to treat severe ankylosing spondylitis and non-radiographic axial spondyloarthritis in adults. You may have received other medicines before. If your response to these medicines is not sufficient, you will be given Yuflyma.
Psoriatic Arthritis
Psoriatic arthritis is an inflammatory disease of the joints that is often associated with psoriasis.
Yuflyma is used to treat psoriatic arthritis in adults. Yuflyma can reduce the damage to your joints caused by the disease and help you move more freely. You may have received other medicines before. If your response to these medicines is not sufficient, you will be given Yuflyma.
Plaque Psoriasis
Plaque psoriasis is a skin disease that causes red, scaly, crusty, and silvery-scaled patches. Plaque psoriasis can also affect the nails, causing them to deteriorate, thicken, and lift off the nail bed, which can be painful.
Yuflyma is used to treat
- chronic plaque psoriasis in adults and
- severe chronic plaque psoriasis in children and adolescents from 4 to 17 years of age who have not responded to or are not candidates for topical treatment and phototherapies.
Hidradenitis Suppurativa
Hidradenitis suppurativa (also known as inverse acne) is a chronic and often painful inflammatory skin disease. Symptoms can include painful nodules (lumps) and abscesses (boils) that can ooze pus. It usually affects specific areas of the skin, such as under the breast, armpits, inner thighs, groin, and buttocks. There may also be scarring in the affected areas.
Yuflyma is used to treat
- moderate to severe hidradenitis suppurativa in adults and
- moderate to severe hidradenitis suppurativa in adolescents from 12 to 17 years of age.
Yuflyma can reduce the number of nodules and abscesses caused by the disease and the pain that is usually associated with this disease. You may have received other medicines before. If your response to these medicines is not sufficient, you will be given Yuflyma.
Crohn's Disease
Crohn's disease is an inflammatory disease of the digestive tract. Yuflyma is used to treat
- moderate to severe Crohn's disease in adults and
- moderate to severe Crohn's disease in children and adolescents from 6 to 17 years of age.
You may have received other medicines before. If your response to these medicines is not sufficient, you will be given Yuflyma.
Ulcerative Colitis
Ulcerative colitis is an inflammatory disease of the large intestine.
Yuflyma is used to treat
- moderate to severe ulcerative colitis in adults and
- moderate to severe ulcerative colitis in children and adolescents from 6 to 17 years of age.
You may have received other medicines before. If your response to these medicines is not sufficient, you will be given Yuflyma.
Non-infectious Uveitis
Non-infectious uveitis is an inflammatory disease that affects certain parts of the eye. Yuflyma is used to treat
- adults with non-infectious uveitis with inflammation that affects the back of the eye and
- children from 2 years of age with chronic non-infectious uveitis with inflammation that affects the front of the eye.
This inflammation can lead to a decrease in vision or the presence of floaters in the eye (black dots or thin lines that move across the field of vision). Yuflyma works by reducing this inflammation.
You may have received other medicines before. If your response to these medicines is not sufficient, you will be given Yuflyma.
2. What you need to know before starting to use Yuflyma
Do not use Yuflyma:
- If you are allergic to adalimumab or to any of the other components of this medicine (included in section 6).
- If you have active tuberculosis or other severe infections (see "Warnings and precautions"). It is important that you inform your doctor if you have symptoms of infection, such as fever, wounds, feeling of tiredness, dental problems.
- If you have moderate or severe heart failure. It is important that you inform your doctor if you have had or have a severe heart condition (see “Warnings and precautions").
Warnings and precautions
Consult your doctor or pharmacist before starting to use Yuflyma.
Allergic reactions
- If you have an allergic reaction with symptoms such as chest tightness, difficulty breathing, dizziness, swelling, or rash, discontinue the administration of Yuflyma and contact your doctor immediately, as in rare cases, these reactions can be life-threatening.
Infections
- If you have any infection, including chronic infection or a localized infection in some part of the body (for example, an ulcer on the leg), consult your doctor before starting treatment with Yuflyma. If you are not sure, contact your doctor.
- You may be more likely to get infections while receiving treatment with Yuflyma. This risk may be greater if you have damaged lungs. These infections can be severe and include:
- tuberculosis
- infections caused by viruses, fungi, parasites, or bacteria
- severe infection in the blood (sepsis)
In rare cases, these infections could be life-threatening. It is essential that if you have symptoms such as fever, wounds, tiredness, or dental problems, you tell your doctor. Your doctor may indicate that you stop using Yuflyma for some time.
- Tell your doctor if you live or travel to regions where fungal infections (such as histoplasmosis, coccidioidomycosis, or blastomycosis) are very common.
- Tell your doctor if you have had recurrent infections or other disorders that increase the risk of infections.
- If you are over 65 years old, you may be more susceptible to infections while being treated with Yuflyma. Both you and your doctor should pay special attention to the appearance of signs of infection while being treated with Yuflyma. It is essential that if you have symptoms of infections such as fever, wounds, tiredness, or dental problems, you tell your doctor.
Tuberculosis
- Since cases of tuberculosis have been reported in patients treated with Yuflyma, your doctor will examine you for signs or symptoms of tuberculosis before starting your treatment with Yuflyma. This will include a thorough medical evaluation, including your medical history and appropriate diagnostic tests (such as chest X-ray and tuberculin test). The performance and results of these tests should be recorded in your patient information card.
- Tuberculosis can develop during treatment, even if you have received treatment to prevent tuberculosis.
- If symptoms of tuberculosis appear (such as persistent cough, weight loss, lack of energy, low-grade fever) or any other infection during or after treatment, contact your doctor immediately.
Hepatitis B
- Tell your doctor if you are a carrier of the hepatitis B virus (HBV), if you have had active HBV infections, or if you think you may be at risk of contracting HBV.
- Your doctor should perform an analysis for HBV. In people carrying HBV, Yuflyma can cause the virus to become active again.
- In rare cases, especially if you are taking other medications that suppress the immune system, the reactivation of HBV can be life-threatening.
Surgery or dental intervention
- If you are going to undergo a surgical or dental intervention, inform your doctor that you are taking Yuflyma. Your doctor may recommend that you temporarily discontinue treatment with Yuflyma.
Demyelinating disease
- If you have or develop a demyelinating disease (a disease that affects the insulation layer around the nerves, such as multiple sclerosis), your doctor will decide whether you should be treated or continue treatment with Yuflyma. Inform your doctor immediately if you experience symptoms such as changes in vision, weakness in arms or legs, or numbness or tingling in any part of the body.
Vaccinations
- Certain vaccines can cause infections and should not be administered if you are being treated with Yuflyma.
- Consult your doctor before receiving any vaccine.
- If possible, it is recommended that children receive the scheduled vaccines for their age before starting treatment with Yuflyma.
- If you have received Yuflyma while pregnant, your baby may be at higher risk of contracting an infection for approximately five months after the last dose of Yuflyma you received during pregnancy. It is essential that you inform your child's doctors and other healthcare professionals about your use of Yuflyma during pregnancy, so they can decide whether your child should receive any vaccine.
Heart failure,
- If you have mild heart failure and are being treated with Yuflyma, your doctor should continuously monitor your heart failure. It is essential that you inform your doctor if you have had or have any severe heart condition. If new symptoms of heart failure appear or existing ones worsen (such as difficulty breathing or swelling of the feet), you should contact your doctor immediately. Your doctor will decide whether you should receive Yuflyma.
Fever, bruising, bleeding, or pale appearance
- In some patients, the body may be unable to produce enough blood cells that help the body fight infections or contribute to stopping bleeding. Your doctor may decide to suspend treatment. If you have persistent fever, easy bruising, bleed easily, or are very pale, consult your doctor immediately.
Cancer
- In very rare cases, certain types of cancer have been reported in children and adults treated with Yuflyma or other TNF-blocking agents.
- People with more severe rheumatoid arthritis and who have had the disease for a long time may have a higher-than-average risk of developing lymphoma (a cancer that affects the lymphatic system) and leukemia (a cancer that affects the blood and bone marrow).
- If you take Yuflyma, the risk of developing lymphoma, leukemia, or other types of cancer may increase. A rare and severe type of lymphoma has been observed in patients treated with Yuflyma. Some of these patients were also receiving treatment with azathioprine or 6-mercaptopurine.
- Tell your doctor if you are taking azathioprine or 6-mercaptopurine with Yuflyma.
- Cases of non-melanoma skin cancer have been observed in patients using Yuflyma.
- Tell your doctor if new skin lesions appear or existing ones change in appearance during or after treatment.
- Cancers other than lymphoma have been reported in patients with a certain lung disease, called Chronic Obstructive Pulmonary Disease (COPD), treated with another TNF-blocking agent. If you have COPD or are a heavy smoker, you should consult your doctor to determine if treatment with a TNF blocker is suitable for you.
Autoimmune disease
- In rare cases, treatment with Yuflyma could lead to a syndrome similar to lupus. Contact your doctor if you have symptoms such as unexplained persistent rash, fever, joint pain, or tiredness.
Children and adolescents
- Vaccination: if possible, your child should be up to date with all vaccinations before using Yuflyma.
Use of Yuflyma with other medications
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication. You should not take Yuflyma with medications that contain the following active substances due to an increased risk of severe infections:
- anakinra
- abatacept.
Yuflyma can be taken with:
- methotrexate
- certain disease-modifying antirheumatic drugs (such as sulfasalazine, hydroxychloroquine, leflunomide, and injectable gold salts)
- corticosteroids or pain medications, including non-steroidal anti-inflammatory drugs (NSAIDs).
If you have any doubts, consult your doctor.
Pregnancy and breastfeeding
- You should consider using adequate contraceptive methods to avoid pregnancy and continue using them for at least 5 months after the last treatment with Yuflyma.
- If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor about the use of this medication.
- Yuflyma should be used during pregnancy only if necessary.
- According to a study in pregnancy, there was no increased risk of congenital defects when the mother had received treatment with Yuflyma during pregnancy compared to mothers with the same disease who did not receive treatment with Yuflyma.
- Yuflyma can be used during breastfeeding.
- If you receive Yuflyma while pregnant, your child may have a higher risk of contracting an infection.
Driving and using machines
Yuflyma may have a small effect on the ability to drive, ride a bicycle, or use machines. You may experience dizziness and vision disturbances after using Yuflyma.
YUFLYMA contains sodium
This medication contains less than 1 mmol of sodium (23 mg) per 0.4 ml dose; this is essentially “sodium-free”
3. How to use Yuflyma
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
In the following table, the recommended doses of Yuflyma are indicated for each of its approved uses. Your doctor may prescribe a different dose of Yuflyma if you need a different dose.
Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, or axial spondyloarthritis without radiographic evidence of ankylosing spondylitis | ||
Age or body weight | How much and how often should it be taken? | Notes |
Adults | 40 mg every two weeks | In rheumatoid arthritis, continue treatment with methotrexate during the use of Yuflyma. If the doctor decides that methotrexate is inappropriate, Yuflyma may be administered as monotherapy. If you have rheumatoid arthritis and are not receiving methotrexate with your treatment with Yuflyma, your doctor may decide to administer 40 mg of Yuflyma every week or 80 mg every two weeks. |
Polyarticular juvenile idiopathic arthritis | ||
Age or body weight | How much and how often should it be taken? | Notes |
Children, adolescents, and adults from 2 years of age with a weight of 30 kg or more | 40 mg every two weeks | Not applicable |
Children and adolescents from 2 years of age with a weight of 10 kg to 30 kg | 20 mg every two weeks | Not applicable |
Arthritis associated with enthesitis | ||
Age or body weight | How much and how often should it be taken? | Notes |
Children, adolescents, and adults from 6 years of age with a weight of 30 kg or more | 40 mg every two weeks | Not applicable |
Children and adolescents from 4 to 17 years of age with a weight of 15 kg to 30 kg | The initial dose is 20 mg followed by 20 mg one week later. Thereafter, the usual dose is 20 mg every other week. | Not applicable |
Plaque psoriasis | ||
Age or body weight | How much and how often should it be taken? | Notes |
Adults | The initial dose is 80 mg (one 80 mg injection), followed by 40 mg every other week, starting one week after the first dose. | If an inadequate response is obtained, the doctor may increase the dose to 40 mg every week or 80 mg every two weeks. |
Children and adolescents from 4 to 17 years of age with a weight of 30 kg or more | The first dose is 40 mg, followed by 40 mg one week later. From then on, the usual dose is 40 mg every two weeks. | Not applicable |
Hidden adenitis suppurativa | ||
Age or body weight | How much and how often should it be taken? | Notes |
Adults | The initial dose is 160 mg (four 40 mg injections in one day or two 40 mg injections per day for two consecutive days), followed by a dose of 80 mg (two 40 mg injections in one day) two weeks later. After two more weeks, continue with a dose of 40 mg every week or 80 mg every two weeks, as prescribed by the doctor. | It is recommended to use an antiseptic daily on the affected areas |
Adolescents from 12 to 17 years of age with a weight of 30 kg or more | The initial dose is 80 mg (two 40 mg injections), followed by 40 mg every other week, starting one week later. | If an inadequate response is obtained with Yuflyma 40 mg every two weeks, the doctor may increase the dose to 40 mg weekly or 80 mg every two weeks. It is recommended to use an antiseptic daily on the affected areas |
Crohn's disease | ||
Age or body weight | How much and how often should it be taken? | Notes |
Children, adolescents, and adults from 6 years of age with a weight of 40 kg or more | The initial dose is 80 mg (one 80 mg injection), followed by 40 mg two weeks later. If a faster response is needed, the doctor may prescribe an initial dose of 160 mg (four 40 mg injections in one day or two 40 mg injections per day for two consecutive days) followed by 80 mg (two 40 mg injections in one day) two weeks later. Thereafter, the usual dose is 40 mg every two weeks. | The doctor may increase the frequency of the dose to 40 mg every week or 80 mg every two weeks. |
Children and adolescents from 6 to 17 years of age who weigh less than 40 kg | The initial dose is 40 mg, followed by 20 mg two weeks later. If a faster response is needed, the doctor may prescribe an initial dose of 80 mg (two 40 mg injections in one day), followed by 40 mg two weeks later. Thereafter, the usual dose is 20 mg every other week. | Your doctor may increase the frequency of the dose to 20 mg every week. |
Ulcerative colitis | ||
Age or body weight | How much and how often should it be taken? | Notes |
Adults | The initial dose is 160 mg (four 40 mg injections in one day or two 40 mg injections per day for two consecutive days), followed by 80 mg (two 40 mg injections in one day) two weeks later. Thereafter, the usual dose is 40 mg every two weeks. | The doctor may increase the frequency of the dose to 40 mg every week or 80 mg every two weeks |
Children and adolescents from 6 years of age with a weight less than 40 kg | First dose of 80 mg (two 40 mg injections in one day), followed by 40 mg (one 40 mg injection) two weeks later. Thereafter, the usual dose is 40 mg every other week. | You should continue using Yuflyma at the usual dose, even after turning 18 years old |
Children and adolescents from 6 years of age with a weight of 40 kg or more | First dose of 160 mg (four 40 mg injections in one day or two 40 mg injections per day for two consecutive days), followed by 80 mg (two 40 mg injections in one day) two weeks later. Thereafter, the usual dose is 80 mg every other week. | You should continue using Yuflyma at the usual dose, even after turning 18 years old |
Non-infectious uveitis | ||
Age or body weight | How much and how often should it be taken? | Notes |
Adults | The initial dose is 80 mg (two 40 mg injections in one day), followed by 40 mg every two weeks, starting one week after the first dose. | Corticosteroids or other medications that affect the immune system may continue to be taken while using Yuflyma. Yuflyma can also be administered alone. |
Children and adolescents from 2 years of age with a weight less than 30 kg | 40 mg every two weeks | The doctor may prescribe an initial dose of 80 mg to be administered one week before starting the usual schedule of 40 mg every two weeks. Yuflyma is recommended for use in combination with methotrexate |
Form and route of administration
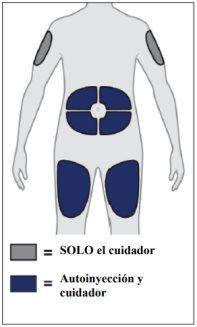
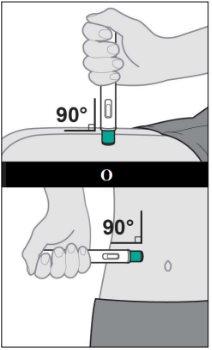
Yuflyma is injected under the skin (subcutaneously).
In section 7 "Instructions for use", detailed instructions are provided on how to inject Yuflyma.
If you use more Yuflyma than you should
If you accidentally inject Yuflyma more frequently than scheduled by your doctor or pharmacist, inform your doctor or pharmacist. Always carry the outer packaging of the medication with you, even if it is empty.
If you forget to use Yuflyma
If you forget to administer an injection, you should inject the next dose of Yuflyma as soon as you remember. Then, the next dose will be administered as usual, as if you had not forgotten a dose.
If you stop treatment with Yuflyma
The decision to stop using Yuflyma should be discussed with your doctor. Symptoms may return if you stop using Yuflyma.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them. Most side effects are mild to moderate. However, some can be serious and require treatment. Side effects may appear at least until 4 months after the last injection of Yuflyma.
Contact your doctor immediately if you notice any of the following effects
- severe rash, hives, or other signs of allergic reaction
- swelling of the face, hands, feet
- difficulty breathing, swallowing
- shortness of breath with physical activity or when lying down or swelling of feet.
Contact your doctor as soon as possible if you notice any of the following effects
- signs of infection, such as fever, nausea, wounds, dental problems, burning sensation when urinating
- feeling of weakness or fatigue
- cough
- tingling
- numbness
- double vision
- weakness in arms or legs
- a lump or an open wound that does not heal
- signs and symptoms of blood disorders such as persistent fever, bruising, bleeding, and paleness
The symptoms described above may be signs of the following side effects, which have been observed with Yuflyma.
Very common(may affect more than 1 person in 10)
- reactions at the injection site (including pain, swelling, redness, or itching)
- respiratory tract infections (including colds, runny nose, sinusitis, pneumonia)
- headache
- abdominal pain
- nausea and vomiting
- skin rash
- musculoskeletal pain
Common(may affect up to 1 in 10 people)
- severe infections (including sepsis and flu)
- intestinal infections (including gastroenteritis),
- skin infections (including cellulitis and herpes)
- ear infections
- oral infections (including dental infection and canker sores)
- infections of the reproductive system
- urinary tract infection
- fungal infections
- joint infections
- benign tumors
- skin cancer
- allergic reactions (including seasonal allergies)
- dehydration
- mood changes (including depression)
- anxiety
- difficulty sleeping
- sensory disturbances such as tingling, itching, or numbness
- migraine
- nerve root compression (including lower back pain and leg pain)
- vision changes
- eye inflammation
- eyelid inflammation and eye swelling
- vertigo (feeling of dizziness or spinning)
- feeling of rapid heartbeat
- high blood pressure
- flushing
- bruising (accumulation of blood outside blood vessels)
- cough
- asthma
- shortness of breath
- gastrointestinal bleeding
- indigestion (indigestion, bloating, and heartburn)
- acid reflux
- dry eye syndrome (including dryness in eyes and mouth)
- itching
- itchy rash
- bruising
- skin inflammation (such as eczema)
- breaking of fingernails and toenails
- increased sweating
- hair loss
- new onset or worsening of existing psoriasis
- muscle spasms
- blood in the urine
- kidney problems
- chest pain
- edema (swelling)
- fever
- decrease in blood platelets, which increases the risk of bleeding or bruising
- Worsening of wound healing
Uncommon(may affect up to 1 in 100 people)
- opportunistic infections (including tuberculosis and other infections that occur when disease resistance decreases)
- neurological infections (including viral meningitis)
- eye infections
- bacterial infections
- diverticulitis (inflammation and infection of the large intestine)
- cancer
- cancer affecting the lymphatic system
- melanoma**
- immune disorders that can affect the lungs, skin, and lymph nodes (the most common presentation is sarcoidosis)
- vasculitis (inflammation of blood vessels)
- tremors (feeling shaky)
- neuropathy (disorder of the nervous system)
- stroke
- hearing loss, ringing in the ears
- feeling of irregular heartbeat like skips
- heart problems that can cause difficulty breathing or swelling of ankles
- heart attack
- bulge in the wall of a major artery, inflammation, and clotting in a vein, blockage of a blood vessel
- lung diseases that can cause difficulty breathing (including inflammation)
- pulmonary embolism (blockage of a lung artery)
- pleural effusion (abnormal accumulation of fluid in the pleural space)
- inflammation of the pancreas that causes severe abdominal and back pain
- difficulty swallowing
- facial edema (swelling of the face)
- inflammation of the gallbladder, gallstones in the gallbladder
- fatty liver
- night sweats
- scarring
- abnormal muscle breakdown
- systemic lupus erythematosus (including skin inflammation, heart, lungs, joints, and other organs)
- sleep disturbances
- impotence
- inflammations
Rare(may affect up to 1 in 1000 people)
- leukemia (cancer that affects the blood and bone marrow)
- severe allergic reaction with shock
- multiple sclerosis
- nervous system disorders (such as optic nerve inflammation and Guillain-Barré syndrome that can cause muscle weakness, abnormal sensations, tingling in the arms and upper body)
- cardiac arrest
- pulmonary fibrosis (scarring in the lungs)
- intestinal perforation (hole in the intestine)
- hepatitis
- reactivation of hepatitis B virus
- autoimmune hepatitis (inflammation of the liver caused by the body's own immune system)
- cutaneous vasculitis (inflammation of blood vessels in the skin)
- Stevens-Johnson syndrome (the first symptoms include discomfort, fever, headache, and rash)
- facial edema (swelling of the face) associated with allergic reactions
- erythema multiforme (inflammatory skin rash)
- lupus-like syndrome
- angioedema (localized skin swelling)
- lichenoid reaction in the skin (red-purple rash with itching)
Frequency not known(cannot be estimated from the available data)
- hepatosplenic T-cell lymphoma (a rare and often fatal blood cancer)
- Merkel cell carcinoma (a type of skin cancer)
- Kaposi's sarcoma, a rare cancer related to human herpesvirus 8 infection. Kaposi's sarcoma usually appears as purple lesions on the skin.
- liver failure
- worsening of a disease called dermatomyositis (seen as a skin rash accompanied by muscle weakness)
- weight gain (for most patients, weight gain was reduced)
Some side effects observed with Yuflyma may not have symptoms and can only be identified through a blood test. These include:
Very common(may affect more than 1 person in 10)
- low white blood cell count
- low red blood cell count
- increased lipids in the blood
- increased liver enzymes
Common(may affect up to 1 in 10 people)
- high white blood cell count
- low platelet count
- increased uric acid in the blood
- abnormal sodium levels in the blood
- low calcium levels in the blood
- low phosphate levels in the blood
- high blood sugar
- high lactate dehydrogenase levels in the blood
- presence of autoantibodies in the blood
- low potassium levels in the blood
Uncommon(may affect up to 1 in 100 people)
- elevated bilirubin levels (liver function test in blood)
Rare(may affect up to 1 in 1000 people)
- low counts in the blood for white cells, red cells, and platelets
Reporting of side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Yuflyma
Keep this medicine out of the sight and reach of children.
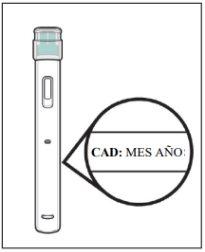
Do not use this medicine after the expiry date which is stated on the carton after “EXP”.
Store in a refrigerator (between 2 °C - 8 °C). Do not freeze.
Keep the pre-filled pen in the outer packaging to protect it from light.
Alternative storage:
When necessary (e.g., when traveling), you may store a pre-filled syringe with a needle guard of Yuflyma at room temperature (up to 25 °C) for a maximum of 31 days (make sure to protect it from light). Once you have removed the syringe from the refrigerator to store it at room temperature, you must use it within the next 31 days or discard it, even if you put it back in the refrigerator.
You should write down the date when you removed the syringe from the refrigerator and the date after which you must discard the syringe.
Medicines should not be disposed of via wastewater or household waste. Ask your doctor how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and additional information
Composition of Yuflyma
The active substance is adalimumab.
The other ingredients are acetic acid, sodium acetate trihydrate, glycine, polysorbate 80, and water for injections.
Appearance of the Yuflyma pre-filled pen and package contents
Yuflyma 40 mg solution for injection in a pre-filled pen is supplied as a sterile solution of 40 mg of adalimumab dissolved in 0.4 ml of solution.
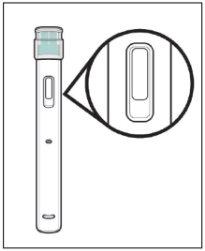
The Yuflyma pre-filled pen is a single-use disposable injection system with automated functions. There is a window on each side of the pen through which you can see the Yuflyma solution inside the pen.
The Yuflyma pre-filled pen is available in packs containing 1, 2, 4, and 6 pre-filled pens.
Each pack of the pre-filled pen contains 2 alcohol swabs (1 spare). Each pack contains 2, 4, or 6 pre-filled pens, and each pre-filled pen is supplied with 1 alcohol swab.
Only some pack sizes may be marketed.
Yuflyma may be available as a pre-filled syringe or pre-filled pen.
Marketing authorisation holder
Celltrion Healthcare Hungary Kft.
1062 Budapest
Váci út 1-3. WestEnd Office Building B torony
Hungary
Manufacturer
Millmount Healthcare Ltd.
Block 7
City North Business Campus
Stamullen, Co. Meath K32 YD60
Ireland
Nuvisan GmbH
Wegenerstraße 13,
89231 Neu Ulm,
Germany
Nuvisan France SARL
2400, Route des Colles,
06410, Biot,
France
You can request more information about this medicine by contacting the local representative of the marketing authorisation holder:
België/Belgique/Belgien Celltrion Healthcare Belgium BVBA Tél/Tel: + 32 1528 7418 | Lietuva Celltrion Healthcare Hungary Kft. Tel.: +36 1 231 0493 |
Celltrion Healthcare Hungary Kft. Teπ.: +36 1 231 0493 | Luxembourg/Luxemburg Celltrion Healthcare Belgium BVBA Tél/Tel: + 32 1528 7418 |
Ceská republika Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 | Magyarország Celltrion Healthcare Hungary Kft. Tel.: +36 1 231 0493 |
Danmark Celltrion Healthcare Hungary Kft. Tlf: +36 1 231 0493 | Malta Mint Health Ltd. Tel: +356 2093 9800 |
Deutschland Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 | Nederland Celltrion Healthcare Netherlands B.V. Tel: + 31 20 888 7300 |
Eesti Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 | Norge Celltrion Healthcare Hungary Kft. Tlf: +36 1 231 0493 |
España Kern Pharma, S.L. Tel: +34 93 700 2525 | Österreich Astro-Pharma GmbH Tel: +43 1 97 99 860 |
Ελλáδα ΒΙΑΝΕΞ Α.Ε. Τηλ: +30 210 8009111 - 120 | Polska Celltrion Healthcare Hungary Kft. Tel.: +36 1 231 0493 |
France Celltrion Healthcare France SAS Tél.: +33 (0)1 71 25 27 00 | Portugal PharmaKERN Portugal - Produtos Farmacêuticos, Sociedade Unipessoal, Lda. Tel: +351 214 200 290 |
Hrvatska Oktal Pharma d.o.o. Tel: +385 1 6595 777 | România Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 |
Ireland Celltrion Healthcare Ireland Limited Tel: +353 1 223 4026 | Slovenija OPH Oktal Pharma d.o.o. Tel.: +386 1 519 29 22 |
Ísland Celltrion Healthcare Hungary Kft. Sími: +36 1 231 0493 | Slovenská republika Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 |
Italia Celltrion Healthcare Italy S.r.l. Tel: +39 0247927040 | Suomi/Finland Celltrion Healthcare Hungary Kft. Puh/Tel: +36 1 231 0493 |
Κúπρος C.A. Papaellinas Ltd Τηλ: +357 22741741 | Sverige Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 |
Latvija Celltrion Healthcare Hungary Kft. Talr.: +36 1 231 0493 | United Kingdom (Nothern Ireland) Celltrion Healthcare Ireland Limited Tel: +353 1 223 4026 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
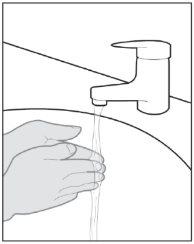
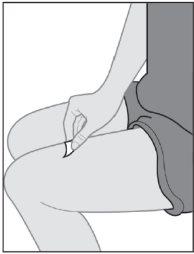
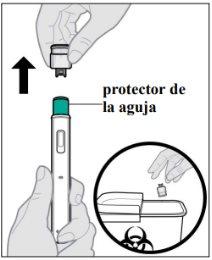
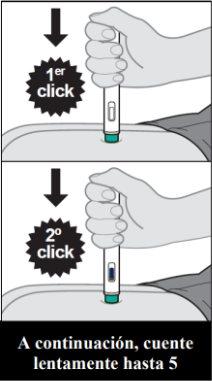
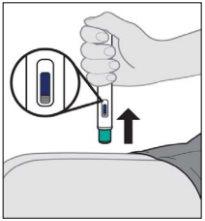
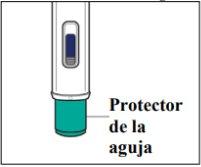
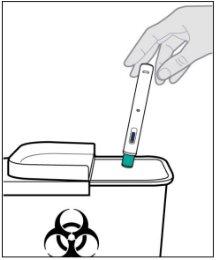
- Instructions for use
- The following instructions explain how to administer a subcutaneous injection of Yuflyma using the pre-filled pen. First, read the instructions carefully and then follow them step by step.
- Your doctor, nurse, or pharmacist will show you the technique for injecting yourself.
- Do notattempt to inject yourself until you are sure you understand how to prepare and administer the injection.
- After you have been properly trained, you or another person, such as a family member or friend, can give the injection.
- Use each pre-filled pen for only one injection.
Yuflyma pre-filled pen
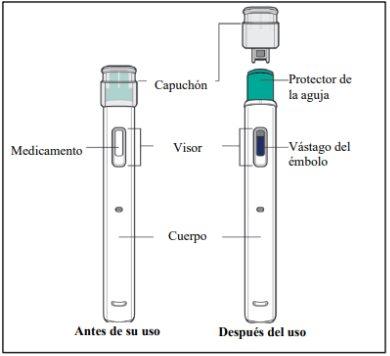
Figure A
Do not use the pre-filled pen if:
- it is cracked or damaged.
- the expiry date has passed.
- it has been dropped onto a hard surface.
Do not remove the cap until you are ready for the injection. Keep Yuflyma out of the reach of children.
Pre-filled pen 1 alcohol swab Not included in the carton:
| |
Figure B |
Do not use the pre-filled pen if:
|
Figure C |
|
Figure D |
|
Figure E |
|
Figure F |
|
Figure G |
|
Figure H |
|
Figure I |
|
Figure J |
|
Figure K
Figure L |
|
Figure M |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to YUFLYMA 40 mg Injectable Solution in a Pre-filled PenDosage form: INJECTABLE, 20 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 20 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 40 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription required
Online doctors for YUFLYMA 40 mg Injectable Solution in a Pre-filled Pen
Discuss questions about YUFLYMA 40 mg Injectable Solution in a Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions