
ZOELY 2.5 mg/1.5 mg FILM-COATED TABLETS

How to use ZOELY 2.5 mg/1.5 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Zoely 2.5mg/1.5mg film-coated tablets
Nomegestrol acetate / Estradiol
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Important things to know about combined hormonal contraceptives (CHCs):
- They are one of the most reliable reversible methods of contraception if used correctly.
- They slightly increase the risk of having a blood clot in the veins and arteries, especially in the first year or when restarting a combined hormonal contraceptive after a break of 4 weeks or more.
- Be alert and see your doctor if you think you might have symptoms of a blood clot (see section 2 “Blood clots”).
Read all of this leaflet carefully before you start takingthismedicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Zoely and what is it used for
- What you need to know before you start using Zoely
- How to use Zoely
- Possible side effects
- Storing Zoely
- Contents of the pack and other information
1. What is Zoely and what is it used for
Zoely is a contraceptive pill used to prevent pregnancy.
- The 24 film-coated tablets, white, are active tablets that contain a small amount of two different female hormones. These are nomegestrol acetate (a progestagen) and estradiol (an estrogen).
- The four yellow tablets are inactive tablets that do not contain hormones and are called placebo tablets.
- Contraceptive pills that contain two different hormones, like Zoely, are called “combined pills”.
- The estradiol, the estrogen in Zoely, is identical to the hormone produced by your ovaries during a menstrual cycle.
- The nomegestrol acetate, the progestagen in Zoely, is derived from progesterone, a hormone that is produced by the ovaries during a menstrual cycle.
2. What you need to know before you start using Zoely
General notes
Before you start using Zoely, you should read the information about blood clots (thrombosis) in section 2. It is particularly important that you read the symptoms of a blood clot (see section 2 “Blood clots”).
Before you start taking Zoely, your doctor will ask you some questions about your personal health and the health of your close relatives. Your doctor will also measure your blood pressure and, depending on your personal situation, may also carry out other tests.
This leaflet describes several situations in which you should either stop using the pill or in which the reliability of the pill may be reduced. In such situations, you should either not have sex or should use additional non-hormonal contraceptive precautions, for example, a condom or another barrier method. Do not use the rhythm or temperature methods. These methods may not be reliable because the pill alters the normal changes in temperature and cervical mucus that occur during the menstrual cycle.
Zoely, like other hormonal contraceptives, does not protect against HIV infection (AIDS) or any other sexually transmitted disease.
When you should not use Zoely
Do not use Zoely if you have any of the conditions listed below. If you have any of the conditions listed below, tell your doctor. Your doctor will discuss with you what other form of contraception would be more suitable.
- If you have (or have ever had) a blood clot in a blood vessel in your legs (deep vein thrombosis, DVT), lungs (pulmonary embolism, PE), or other organs.
- If you know you have a disorder that affects blood clotting: for example, protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden, or antiphospholipid antibodies.
- If you need an operation or if you are going to be immobile for a long time (see section “Blood clots”).
- If you have ever had a heart attack or stroke.
- If you have (or have ever had) angina pectoris (a condition that causes severe chest pain and may be a sign of an impending heart attack) or a transient ischaemic attack (TIA, temporary stroke-like symptoms).
- If you have any of the following diseases that may increase your risk of a blood clot in the arteries.
- severe diabetes with blood vessel damage;
- very high blood pressure;
- very high levels of fat in the blood (cholesterol or triglycerides);
- a condition known as hyperhomocysteinaemia.
- If you have (or have ever had) a type of migraine called “migraine with aura”.
- If you have (or have had) an inflammation of the pancreas (pancreatitis) associated with high levels of fat in the blood.
- If you have (or have had) a severe liver disease and your liver is not yet working normally.
- If you have (or have had) a benign or malignant liver tumour.
- If you have (or have had) or may have a breast or genital cancer.
- If you have any vaginal bleeding that you do not know the cause of.
- If you are allergic to estradiol, nomegestrol acetate, or any of the other ingredients of this medicine (listed in section 6).
If any of these conditions occur for the first time while using Zoely, stop using it immediately and consult your doctor. In the meantime, use a non-hormonal contraceptive. See also “General notes” in section 2, above.
When to be extra cautious when using Zoely
When should you contact your doctor?
Seek urgent medical attention
- If you notice any possible signs of a blood clot that may mean you are experiencing a blood clot in the leg (i.e. deep vein thrombosis), a blood clot in the lung (i.e. pulmonary embolism), a heart attack, or a stroke (see section “Blood clot” below).
To get a description of the symptoms of these serious side effects, see “How to recognize a blood clot”.
- If you notice any changes in your health that may affect any of the items mentioned in this leaflet (see also section 2, “When not to use Zoely”; do not forget changes in the health of your immediate family).
- If you feel a lump in your breast.
- If you experience symptoms of angioedema, such as swelling of the face, tongue, or throat, or difficulty swallowing, or hives, accompanied by difficulty breathing.
- If you are going to use other medicines (see section 2, “Using Zoely with other medicines”).
- If you are going to have an operation or be immobile for a long time (tell your doctor at least 4 weeks in advance).
- If you experience unusual or heavy vaginal bleeding.
- If you have forgotten to take one or more tablets in the first week of the blister pack and have had sex without protection in the past 7 days (see also section 3, “If you forget to take Zoely”).
- If you have severe diarrhoea or severe vomiting.
- If you have missed a period and suspect you are pregnant (do not start the next blister pack until your doctor tells you to; see also section 3, “If you have missed one or two periods”).
Tell your doctor if you have any of the following conditions.
If the condition develops or worsens while you are using Zoely, you should also tell your doctor.
- If you have hereditary angioedema. See your doctor immediately if you experience symptoms of angioedema, such as swelling of the face, tongue, or throat, or difficulty swallowing, or hives, accompanied by difficulty breathing. Medicines that contain estrogens may induce or worsen the symptoms of hereditary angioedema.
- If a close relative has had breast cancer.
- If you have epilepsy (see section 2, “Using Zoely with other medicines”).
- If you have a liver disease (e.g. jaundice) or gallbladder disease (e.g. gallstones).
- If you have diabetes.
- If you have depression.
- If you have Crohn’s disease or ulcerative colitis (chronic inflammatory bowel disease).
- If you have systemic lupus erythematosus (SLE, a disease that affects your natural defence system).
- If you have haemolytic uraemic syndrome (HUS, a blood clotting disorder that causes kidney failure).
- If you have sickle cell anaemia (a hereditary disease of the red blood cells).
- If you have high levels of fat in the blood (hypertriglyceridaemia) or a known family history of this condition. Hypertriglyceridaemia has been associated with an increased risk of pancreatitis (inflammation of the pancreas).
- If you need an operation or are going to be immobile for a long time (see section 2 “Blood clots”).
- If you have just given birth, you are at a higher risk of blood clots. You should ask your doctor how soon after delivery you can start taking Zoely.
- If you have inflammation of the veins just under the skin (superficial thrombophlebitis).
- If you have varicose veins.
- If you have a condition that first occurred or worsened during pregnancy or previous use of sex hormones; for example, loss of hearing, porphyria (a blood disorder), gestational herpes (a blister-like rash during pregnancy), Sydenham's chorea (a nerve disease that causes sudden movements of the body), angioedema (hereditary). (See section 2, “When to contact your doctor”.)
- If you have (or have ever had) chloasma (brown-dorado patches, known as “pregnancy patches”, mainly on the face). In this case, avoid excessive exposure to the sun or ultraviolet light.
BLOOD CLOTS
The use of a combined hormonal contraceptive like Zoely increases your risk of having a blood clot compared to not using it. In rare cases, a blood clot can block blood vessels and cause serious problems.
Blood clots can form:
- In the veins (this is called "venous thrombosis", "venous thromboembolism" or VTE).
- In the arteries (this is called "arterial thrombosis", "arterial thromboembolism" or ATE).
Recovery from blood clots is not always complete. In rare cases, there can be serious long-lasting effects or, very rarely, they can be fatal.
It is important to remember that the overall risk of having a harmful blood clot due to Zoely is small.
HOW TO RECOGNIZE A BLOOD CLOT
Seek medical attention immediately if you notice any of the following signs or symptoms.
Are you experiencing any of these signs? | What might you be suffering from? |
| Deep vein thrombosis |
If you are unsure, consult a doctor, as some of these symptoms, such as coughing or shortness of breath, can be confused with a milder condition like a respiratory infection (e.g. a "common cold"). | Pulmonary embolism |
Symptoms that occur more frequently in one eye:
| Retinal vein thrombosis (blood clot in the eye) |
| Heart attack |
Sometimes the symptoms of a stroke can be brief, with almost immediate and complete recovery, but you should still seek medical attention urgently as you may be at risk of having another stroke. | Stroke |
| Blood clots that block other blood vessels |
BLOOD CLOTS IN A VEIN
What can happen if a blood clot forms in a vein?
- The use of combined hormonal contraceptives has been associated with an increased risk of blood clots in the veins (venous thrombosis). However, these adverse effects are rare. They occur more frequently in the first year of use of a combined hormonal contraceptive.
- If a blood clot forms in a vein in the leg or foot, it can cause deep vein thrombosis (DVT).
- If a blood clot moves from the leg and lodges in the lung, it can cause a pulmonary embolism.
- In very rare cases, a clot can form in a vein of another organ, such as the eye (retinal vein thrombosis).
When is the risk of having a blood clot in a vein higher?
The risk of having a blood clot in a vein is higher during the first year you take a combined hormonal contraceptive for the first time. The risk may also be higher if you start taking a combined hormonal contraceptive (the same product or a different product) again after a break of 4 weeks or more.
After the first year, the risk decreases, but it is always slightly higher than if you were not taking a combined hormonal contraceptive.
When you stop taking Zoely, your risk of having a blood clot returns to normal within a few weeks.
What is the risk of having a blood clot?
The risk depends on your natural risk of VTE and the type of combined hormonal contraceptive you are taking.
The overall risk of having a blood clot in the leg or lung (DVT or PE) with Zoely is small.
- Out of 10,000 women who do not use a combined hormonal contraceptive and are not pregnant, about 2 will have a blood clot in a year.
- Out of 10,000 women who use a combined hormonal contraceptive that contains levonorgestrel, norethisterone, or norgestimate, about 5-7 will have a blood clot in a year.
- It is not yet known how the risk of a blood clot with Zoely compares to the risk with a combined hormonal contraceptive that contains levonorgestrel.
- The risk of having a blood clot will vary depending on your personal medical history (see "Factors that increase your risk of a blood clot" below).
Risk of having a blood clot in a year | |
Women who do not usea combined hormonal contraceptive and who are not pregnant | About 2 out of 10,000 women |
Women who use a combined hormonal contraceptive that contains levonorgestrel, norethisterone, or norgestimate | About 5-7 out of 10,000 women |
Women who use Zoely | Not yet known |
Factors that increase your risk of a blood clot in a vein
The risk of having a blood clot with Zoely is small, but some conditions increase the risk. Your risk is higher:
- If you are overweight (body mass index or BMI over 30 kg/m2).
- If any of your close relatives have had a blood clot in the leg, lung, or other organ at a young age (i.e. before the age of about 50).
- If you need to have surgery or if you are immobile for a long time due to an injury or illness, or if you have a leg in a cast. You may need to stop taking Zoely several weeks before the surgery or while you are less mobile. If you need to stop taking Zoely, ask your doctor when you can start taking it again.
- As you get older (especially above about 35 years).
- If you have recently given birth.
The risk of having a blood clot increases the more conditions you have.
Long-distance flights (more than 4 hours) may temporarily increase the risk of a blood clot, especially if you have any of the other risk factors listed.
It is important to tell your doctor if you have any of the conditions listed above, even if you are not sure. Your doctor may decide that you should stop taking Zoely.
If any of the conditions listed above change while you are taking Zoely, for example, you start smoking, a close relative has a blood clot without a known cause, or you gain a lot of weight, tell your doctor.
BLOOD CLOTS IN AN ARTERY
What can happen if a blood clot forms in an artery?
Like a blood clot in a vein, a clot in an artery can cause serious problems. For example, it can cause a heart attack or a stroke.
Factors that increase your risk of a blood clot in an artery
It is important to note that the risk of a heart attack or stroke due to Zoely is very small, but it can increase:
- With age (above about 35 years).
- If you smoke. When using a combined hormonal contraceptive like Zoely, you are advised to stop smoking. If you are unable to stop smoking and are over 35 years old, your doctor may advise you to use a different type of contraceptive.
- If you are overweight.
- If you have high blood pressure.
- If any of your close relatives have had a heart attack or stroke at a young age (less than about 50 years). In this case, you may also be at higher risk of having a heart attack or stroke.
- If you or any of your close relatives have high levels of fat in the blood (cholesterol or triglycerides).
- If you have migraines, especially migraines with aura.
- If you have a heart problem (valve disorder, a heart rhythm disorder called atrial fibrillation).
- If you have diabetes.
If you have one or more of these conditions or if any of them are particularly severe, the risk of having a blood clot may be increased further.
If any of the conditions listed above change while you are taking Zoely, for example, you start smoking, a close relative has a blood clot without a known cause, or you gain a lot of weight, tell your doctor.
CANCER
Cases of breast cancer have been observed with slightly higher frequency in women taking combined pills, but it is not known if this is caused by the combined pills. For example, it may be that more tumors are detected in women taking combined pills because they are examined by their doctor more often. After stopping the combined pill, the increased risk decreases gradually.
It is important that you have your breasts checked regularly and consult your doctor if you notice any lump. You should also inform your doctor if a close relative has had or has breast cancer (see section 2, "When you should have special care with Zoely").
In rare cases, benign (non-cancerous) liver tumors and, even more rarely, malignant (cancerous) liver tumors have been reported in women taking pills. Contact your doctor if you experience exceptionally severe abdominal pain.
Cervical cancer is caused by a human papillomavirus (HPV) infection. It has been reported that it occurs more frequently in women who use the pill for more than 5 years. It is not known if this observation is due to the use of hormonal contraceptives or to other factors, such as differences in sexual behavior.
Laboratory Tests
If you have any blood or urine tests, inform your doctor that you are taking Zoely, as it may affect the results of some tests.
Children andAdolescents
No data are available on the safety and efficacy in adolescents under 18 years of age.
Use of Zoely with Other Medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those bought without a prescription and herbal medicines.
Also, inform any other doctor or dentist prescribing you another medicine (or the pharmacist dispensing the medicine) that you are taking Zoely. They may tell you that you need to take extra precautions (a barrier method) and, if so, for how long.
- There are medicines that may make Zoely less effective in preventing pregnancy or may cause unexpected bleeding. These include medicines for:
- epilepsy (e.g. primidone, phenytoin, phenobarbital, carbamazepine, oxcarbazepine, topiramate, felbamate);
- tuberculosis (e.g. rifampicin);
- HIV infection (e.g. ritonavir, nevirapine, nelfinavir, efavirenz);
- other infectious diseases (e.g. griseofulvin);
- high blood pressure in the blood vessels of the lungs (bosentan).
- The herbal medicine called St. John's Wort or hypericum may also make Zoely less effective. If you want to use products containing St. John's Wort while you are taking Zoely, you should consult your doctor first.
- Some medicines may increase the levels of the active substances of Zoely in the blood. The effectiveness of the pill is maintained, but inform your doctor if you are using medicines for fungal infections that contain ketoconazole.
- Zoely may also interfere with the function of other medicines, such as the antiepileptic called lamotrigine.
Pregnancy and Breastfeeding
Pregnant or potentially pregnant women should not use Zoely. If you become pregnant while taking Zoely, you should stop taking it and contact your doctor.
If you want to stop taking Zoely because you want to become pregnant, see section 3, "If you stop taking Zoely".
Zoely is not generally recommended during breastfeeding. If you want to use the pill while breastfeeding, consult your doctor.
Consult your doctor or pharmacist before taking any medicine.
Driving and Using Machines
Zoely has no or negligible influence on the ability to drive or use machines.
Zoelycontains lactose
Zoely contains lactose. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
3. How to use Zoely
When and how to take the tablets
The Zoely blister pack contains 28 tablets: 24 white tablets with active ingredients (numbers 1 to 24) and four yellow tablets without active ingredients (numbers 25 to 28).
Each time you start a new Zoely blister pack, take the white active tablet, identified with the number 1, in the top left corner (see "Start"). Choose from the seven stickers with day indicators, the one in the gray column that starts with your start day. For example, if you start on a Wednesday, use the day-of-the-week sticker that says "WED". Stick it on the blister pack, just above the row of white active tablets, where it says "Place day sticker here". This allows you to check if you took your daily tablet.
Take one tablet every day, approximately at the same time; if necessary, with a little water.
Follow the direction of the arrows on the blister pack, so use the white active tablets first, and then the yellow placebo tablets.
Your menstruation will begin during the four days you take the yellow placebo tablets (this menstruation is called withdrawal bleeding). It usually starts two to three days after the last white active tablet and may not have ended before starting the next blister pack.
Start taking the next blister pack immediately after the last yellow tablet, even if your menstruation has not ended. This means you will always start a new blister pack on the same day of the week, and you will also have your menstruation approximately on the same days every month.
Some users may not have menstruation every month while taking the yellow tablets. If you have taken Zoely every day according to these instructions, it is unlikely that you are pregnant (see also section 3, "If you have missed one or two menstruations").
Start of your first Zoely pack
If you have not used any hormonal contraceptive in the previous month
Start taking Zoely on the first day of your cycle (i.e., the first day of your menstruation). Zoely will work immediately. You do not need to use an additional contraceptive method.
If you are switching from another combined hormonal contraceptive (combined pill, vaginal ring, or transdermal patch)
You can start taking Zoely the day after you took the last tablet from your current pill pack (this means there is no interruption in taking the tablets). If your current pill pack also contains inactive tablets (placebo), you can start Zoely the day after you take the last activetablet (if you are not sure which one it is, ask your doctor or pharmacist). You can also start later, but never after the day following the interruption of taking the pills you are taking now (or the day after the last inactive tablet of your current pill). If you are using a vaginal ring or transdermal patch, it is best to start using Zoely on the day you remove the ring or patch. You can also start, at the latest, on the day you would have started using the next ring or patch.
If you follow these instructions, you do not need to use an additional contraceptive method.
If you are switching from a progestin-only pill (minipill)
You can stop taking the minipill on any day and start taking Zoely the next day. But if you have had sex, make sure to use a barrier contraceptive method during the first seven days you are taking Zoely.
If you are switching from a progestin-only injection, implant, or hormone-releasing intrauterine system
Start using Zoely when your next injection is due or on the day the implant or intrauterine system is removed. But if you have had sex, make sure to use a barrier contraceptive method during the first seven days you are taking Zoely.
After having a baby
You can start taking Zoely between 21 and 28 days after giving birth. If you start after the 28th day, you should also use a barrier contraceptive method during the first seven days you use Zoely. If, after giving birth, you have had sex before starting to take Zoely, make sure you are not pregnant or wait until your next menstruation. If you want to start taking Zoely after giving birth and are breastfeeding, also read section 2 "Pregnancy and breastfeeding".
If you are not sure when to start, consult your doctor.
After a spontaneous or induced abortion
Follow your doctor's advice.
If you take more Zoely than you should
There have been no reports of serious harmful effects from taking too many Zoely tablets at once. If you have taken several tablets at once, you may have nausea, vomiting, or vaginal bleeding. If you discover that a child has taken Zoely, ask your doctor for advice.
If you forget to take Zoely
The following advice only refers to the white activetablets that you have forgotten to take.
- If less than 24hourshave passed since the time you forgot to take a tablet, the reliability of the pill is maintained. Take the tablet as soon as you remember and then take the following tablets at the usual time.
- If 24hours or morehave passed since the time you forgot to take a tablet, the reliability of the pill may be reduced. The more consecutive tablets you have forgotten, the higher the risk that the contraceptive effectiveness is reduced. There is a particularly high risk of becoming pregnant if you forget to take whiteactive tablets at the beginning or end of the blister pack. Therefore, you should follow the instructions below.
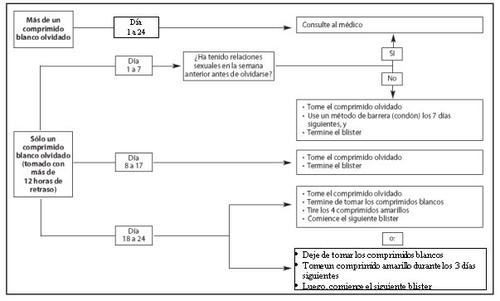
Day1 to 7 of taking the white active tablets (see figure and scheme)
Take the last white active tablet you forgot to take as soon as you remember (even if this means taking two tablets at once) and take the next tablet at the usual time. However, use a barrier contraceptive method, for example, a condom, as an additional precaution until you have taken the tablets correctly for seven consecutive days.
If you have had sex in the week before your missed tablet, there is a possibility that you may become or are pregnant. Consult your doctor immediately.
Day8 to 17 of taking the white active tablets (see figure and scheme)
Take the last tablet you forgot to take as soon as you remember (even if this means taking two tablets at once) and take the following tablets at the usual time. If you have taken the tablets correctly during the seven days before the missed tablet, the protection against pregnancy is not decreased, and you do not need to take additional precautions. However, if you have forgotten to take more than one tablet, use a barrier contraceptive method, for example, a condom, as an additional precaution until you have taken the tablets correctly for seven consecutive days.
Day18 to 24 of taking the white active tablets (see figure and scheme)
There is a particularly high risk of becoming pregnant if you forget to take white active tablets near the interval of the yellow placebo tablets. This higher risk can be avoided if you adjust your tablet-taking schedule.
You can follow the two options indicated below. You do not need to take additional precautions if you have taken the tablets correctly during the seven days before the missed tablet. If not, you should follow the first of the two options and use a barrier contraceptive method, for example, a condom, as an additional precaution until you have taken the tablets correctly for seven consecutive days.
1st option:
Take the last white active tablet you forgot to take as soon as you remember (even if this means taking two tablets at once) and take the following tablets at the usual time. Start the next blister pack as soon as you finish the white active tablets of the current blister pack, i.e., skip the yellow placebo tablets. You may not have your menstruation until you take the yellow placebo tablets at the end of the second blister pack, but you may have oligomenorrhea (drops or spots of blood) or intermenstrual bleeding while taking the white active tablets.
2nd option:
Stop taking the white active tablets and start taking the yellow placebo tablets for up to three days so that the total number of placebo tablets plus white active tablets is not more than four. At the end of this interval, start the next blister pack.
If you cannot remember how many white active tablets you have forgotten to take, follow the first option, use a barrier contraceptive method, for example, a condom, as an additional precaution until you have taken the tablets correctly for seven consecutive days, and consult your doctor (since it is possible that you were not protected against pregnancy).
If you have forgotten to take white active tablets from a blister pack and do not have your expected monthly menstruation while taking the yellow placebo tablets from the same blister pack, you may be pregnant. Consult your doctor before starting the next Zoely blister pack.
If you forgot the yellow placebo tablets
The four last yellow tablets in the fourth row are placebo tablets that do not contain active ingredients. If you have forgotten to take one of these tablets, the reliability of Zoely is maintained. Discard the yellow placebo tablet(s) you forgot to take and continue taking the following tablets at the usual time.
Figure
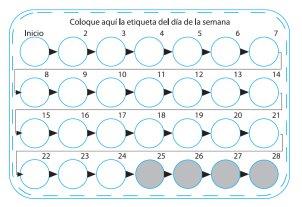
Calendar: If 24 hours or more have passed since you were supposed to take the white tablets


If you vomit or have severe diarrhea
If you vomit within three or four hours after taking a white active tablet, or if you have severe diarrhea, the active ingredients of your Zoely tablet may not have been fully absorbed into your body. This situation is similar to if you forget to take a white active tablet. After vomiting or having diarrhea, you should take, as soon as possible, a white active tablet from a reserve blister pack. If possible, take it within 24hoursof the time you normally take the pill. Take the next tablet at the usual time. If this is not possible or if 24 hours or more have passed, you should follow the recommendation under "If you forget to take Zoely". If you have severe diarrhea, consult your doctor.
The yellow tablets are placebos and do not contain active ingredients. If you vomit or have severe diarrhea within three to four hours after taking a yellow tablet, the reliability of Zoely is maintained.
If you want to delay your menstruation
You can delay your menstruation by not taking the yellow placebo tablets and going directly to a new Zoely blister pack. You may have light bleeding or bleeding similar to menstruation while using this second blister pack. When you want your menstruation to begin during the second blister pack, stop taking the white active tablets and start taking the yellow placebo tablets. After finishing the four yellow placebo tablets of the second blister pack, start with the next blister pack (the third).
If you want to change the day of your menstruation
If you take the tablets according to the instructions, your menstruation will begin during the days you take the placebo. If you need to change this day, reduce the number of days you take the placebo, i.e., when you take the yellow placebo tablets (but never increase them; the maximum is four). For example, if you start taking the placebo tablets on Friday and want to change it to Tuesday (three days earlier), you should start a new blister pack three days earlier than usual. You may not have bleeding during the shortened period you take the yellow placebo tablets. While using the next blister pack, you may have some oligomenorrhea (drops or spots of blood) or intermenstrual bleeding on the days you take the white active tablets.
If you are not sure what to do, consult your doctor.
If you have unexpected bleeding
With all combined pills, during the first months, you may have some irregular vaginal bleeding (oligomenorrhea or intermenstrual bleeding) between menstruations. You may need to use sanitary protection, but continue taking the tablets as usual. The irregular vaginal bleeding usually stops once your body has adjusted to the pill (usually after about three months). If the bleeding continues, becomes heavy, or starts again, consult your doctor.
If you have missed one or more menstruations
In clinical studies with Zoely, it has been observed that it is possible that, occasionally, you may not have regular monthly menstruation after the 24th day.
- If you have taken all the tablets correctly, have not had vomiting or severe diarrhea, and have not taken any other medication, then it is very unlikely that you are pregnant. Continue taking Zoely as usual. Also, consult section 3, "If you vomit or have diarrhea"or section 2, "Using Zoely with other medicines".
- If you have nottaken the tablets correctly or if the expected menstruation is missing two times in a row, you may be pregnant. Contact your doctor immediately. Do not start the next Zoely blister pack until your doctor has checked that you are not pregnant.
If you stop taking Zoely
You can stop taking Zoely at any time. If you do not want to become pregnant, ask your doctor about other contraceptive methods first.
If you have stopped taking Zoely because you want to become pregnant, it is recommended that you wait until you have had a natural menstruation before trying to conceive. This will help you determine when the baby is due to be born.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If you experience any adverse effect, especially if it is severe and persistent, or have any change in health that you think may be due to Zoely, consult your doctor.
All women who take combined hormonal contraceptives are at a higher risk of developing blood clots in the veins (venous thromboembolism [VTE]) or blood clots in the arteries (arterial thromboembolism [ATE]). For more detailed information on the different risks of taking combined hormonal contraceptives, see section 2 “What you need to know before you start using Zoely”.
The following adverse effects have been associated with the use of Zoely:
Very common (may affect more than 1 in 10 people):
- acne;
- changes in menstruation (e.g., absence or irregularity);
Common (may affect up to 1 in 10 people):
- decreased sexual desire, depression or depressive mood, mood changes;
- headache or migraine;
- feeling sick (nausea);
- heavy menstruation, breast pain, pelvic pain;
- weight gain.
Uncommon (may affect up to 1 in 100 people):
- increased appetite, fluid retention (edema);
- hot flashes;
- abdominal bloating;
- increased sweating, hair loss, itching, dry skin, oily skin;
- heaviness in the limbs;
- regular but scanty menstruation, breast enlargement, breast lump, milk production while not pregnant, premenstrual syndrome, painful intercourse, vaginal or vulvar dryness;
- irritability;
- increased liver enzymes.
Rare (may affect up to 1 in 1,000 people):
- harmful blood clots in a vein or artery, for example:
- in a leg or foot (i.e., DVT);
- in a lung (i.e., PE);
- heart attack;
- stroke;
- mild stroke or temporary symptoms similar to those of a stroke, called a transient ischemic attack (TIA);
- blood clots in the liver, stomach/intestine, kidneys, or eye.
The risk of having a blood clot may be higher if you have any other condition that increases this risk (see section 2 for more information on conditions that increase the risk of blood clots and symptoms of a blood clot).
- decreased appetite;
- increased sexual desire;
- attention disorder;
- dry eyes, intolerance to contact lenses;
- dry mouth;
- brown-gold pigmented patches, especially on the face; excessive hair growth
- vaginal odor, discomfort in the vagina or vulva;
- hunger;
- gallbladder disease.
Allergic reactions (hypersensitivity) have been reported in users of Zoely, but their frequency cannot be estimated from the available data.
More information about changes in menstruation (e.g., absent or irregular) as a possible adverse effect during the use of Zoely can be found in section 3, “When and how to take the tablets”, “If you have unexpected bleeding” and “If you have missed one or more menstruations”.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can help provide more information on the safety of this medicine.
5. Storage of Zoely
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the blister and carton, after EXP/CAD. The expiry date is the last day of the month stated.
No special storage conditions are required.
Combined pills (including Zoely tablets) that are no longer needed should not be disposed of via wastewater or the municipal sewage system. The hormonal active substances in the tablet may have harmful effects if they enter the aquatic environment. Return them to the pharmacy or dispose of them in another safe way, in accordance with local regulations. This will help protect the environment.
6. Package Contents and Additional Information
Zoely Composition
- The active substance(s) is (are): nomegestrol acetate and estradiol. Active film-coated tablets are white: each tablet contains 2.5 mg of nomegestrol acetate and 1.5 mg of estradiol (as hemihydrate). Inactive film-coated tablets are yellow: the tablet does not contain active substances.
- The other ingredients are: Tablet core (active film-coated white tablets and yellow inactive tablets): lactose monohydrate (see section 2, “Zoely contains lactose”), microcrystalline cellulose (E460), crospovidone (E1201), talc (E553b), magnesium stearate (E572), and anhydrous colloidal silica. Tablet coating (active film-coated white tablets): polyvinyl alcohol (E1203), titanium dioxide (E171), macrogol 3350, and talc (E553b). Tablet coating (inactive film-coated yellow tablets): polyvinyl alcohol (E1203), titanium dioxide (E171), macrogol 3350, talc (E553b), yellow iron oxide (E172), and black iron oxide (E172).
Product Appearance and Package Contents
The active film-coated tablets (tablets) are white and round, and have the code “ne” on both sides.
The inactive film-coated tablets are yellow and round, and have the code “p” on both sides.
Zoely is available in blisters of 28 film-coated tablets (24 active film-coated white tablets and 4 inactive film-coated yellow tablets), packaged in a cardboard box.
Package sizes: 28, 84, 168, and 364 film-coated tablets.
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Teva B.V.
Swensweg 5
2031 GA Haarlem
Netherlands
Manufacturer
Organon (Ireland) Limited
Drynam Road
Swords
Co. Dublin
Ireland
Delpharm Lille S.A.S.
Parc d’Activités Roubaix-Est
22 Rue de Toufflers
CS 50070,
59452 LYS-LEZ-LANNOY France
Teva Operations Poland Sp. z o.o.
ul. Mogilska 80
31-546 Krakow
Poland
N.V. Organon
Kloosterstraat 6
5349 AB Oss
Netherlands
Merck Sharp & Dohme B.V.
Waarderweg 39
2031 BN Haarlem
Netherlands
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Teva Pharma Belgium N.V./S.A./AG Tel/Tél: + 32 (0)38 20 73 73 | Lietuva UAB Merck Sharp & Dohme Tel: + 370 5 2780247 |
България Actavis EAD Tel: +359 2 489 95 85 | Luxembourg/Luxemburg Teva Pharma Belgium N.V./S.A./AG Tel/Tél: + 32 (0)38 20 73 73 |
Česká republika Merck Sharp & Dohme s.r.o. Tel.: +420 233 010 111 | Magyarország MSD Pharma Hungary Kft. Tel.: + 36 1 888-5300 |
Danmark MSD Danmark ApS Tlf: + 45 4482 4000 | Malta Merck Sharp & Dohme Cyprus Limited Tel: 8007 4433 (+356 99917558) |
Deutschland MSD SHARP & DOHME GMBH Tel: 0800 673 673 673 (+49 (0) 89 4561 2612) | Nederland Merck Sharp & Dohme BV Tel: 0800 9999 000 (+ 31 23 515 3153) |
Eesti Merck Sharp & Dohme OÜ Tel: + 372 6144 200 | Norge MSD (Norge) AS Tlf: + 47 32 20 73 00 |
Ελλάδα Specifar ABEE Τηλ: +30 210 5401500 | Österreich Merck Sharp & Dohme Ges.m.b.H. Tel: +43 (0) 1 26 044 |
España Teva Pharma S.L.U Tel: +34 91 387 32 80 | Polska MSD Polska Sp. z o.o. Tel.: +48 22 549 51 00 |
France TEVA SANTÉ Tél: + 33 1 55 91 78 00 | Portugal Merck Sharp & Dohme, Lda Tel: + 351 21 4465700 |
Hrvatska Pliva Hrvatska d.o.o. Tel: +385 1 37 20 000 | România Teva Pharmaceuticals S.R.L. Tel: + 4021 230 65 24 |
Ireland Merck Sharp & Dohme Ireland (Human Health) Limited Tel: +353 (0)1 2998700 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 1 58 90 390 |
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika Merck Sharp & Dohme, s.r.o. Tel: + 421 (2) 58282010 |
Italia Teva Italia S.r.l. Tel: +39 02 8917981 | Suomi/Finland MSD Finland Oy Puh/Tel: + 358 (0)9 804650 |
Κύπρος Specifar ABEE, Ελλάδα Τηλ: +30 210 5401500 | Sverige Merck Sharp & Dohme (Sweden) AB Tfn: + 46 (0)77 570 04 88 |
Latvija SIA Merck Sharp & Dohme Latvija Tel: + 371 67 364224 | United Kingdom Merck Sharp & Dohme Limited Tel: +44 (0) 1992 467272 |
Date of Last Revision of this Leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZOELY 2.5 mg/1.5 mg FILM-COATED TABLETSDosage form: TABLET, 2.5mg nomegestrol acetate/1.5mg estradiol hemihydrateActive substance: nomegestrol and estradiolManufacturer: Theramex Ireland LimitedPrescription requiredDosage form: TABLET, 2.5mg nomegestrol acetate/1.5mg estradiol hemihydrateActive substance: nomegestrol and estradiolManufacturer: Theramex Ireland LimitedPrescription requiredDosage form: TABLET, 2 mg/0.03 mgActive substance: dienogest and ethinylestradiolManufacturer: Laboratorios Cinfa S.A.Prescription required
Online doctors for ZOELY 2.5 mg/1.5 mg FILM-COATED TABLETS
Discuss questions about ZOELY 2.5 mg/1.5 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






