ZENNUX 10 mg/ml injectable solution in pre-filled syringe


How to use ZENNUX 10 mg/ml injectable solution in pre-filled syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Zennux 10 mg/ml solution for injection in pre-filled syringe
suxamethonium chloride anhydrous
Read all of this leaflet carefully before this medicine is administered to you because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What Zennux is and what it is used for
- What you need to know before Zennux is administered to you
- How Zennux is administered
- Possible side effects
- Storage of Zennux
- Contents of the pack and further information
1. What Zennux is and what it is used for
Zennux belongs to a group of medicines called muscle relaxants.
Its effect is to block the connection between the nerves and certain muscles, which relaxes these muscles by temporarily paralyzing them.
It is used in adults and in the pediatric population over 12 years of age in general anesthesia or emergency cases.
Zennux is administered during the insertion of a tube into the trachea (endotracheal intubation), if a person needs assistance to breathe. During this procedure, it is necessary that the muscles used for breathing are paralyzed.
2. What you need to know before Zennux is administered to you
Zennux must not be administered:
- if you are allergic to suxamethonium or any of the other ingredients of this medicine (listed in section 6).
- if you are a conscious patient;
- if you or a family member have had a negative reaction to a previous anesthetic like a very high body temperature (malignant hyperthermia);
- if you have a deficiency of an enzyme called pseudocholinesterase that breaks down suxamethonium in the body;
- if you have a high level of potassium in the blood (hyperkalemia);
- if you have had a severe accident, surgery, or severe burns;
- if you have suffered a spinal cord injury, nerve damage, or sudden muscle loss;
- if you have been immobilized for a long period, such as for the treatment of a bone fracture or a long period of bed rest;
- if you have muscle weakness or muscle tissue loss (e.g., Duchenne muscular dystrophy);
- if you or a family member have a disease that causes muscle weakness (congenital myotonia, myotonic dystrophy);
- if you have recently had eye injuries;
- if you suffer from a problem caused by excessive pressure in the eye (glaucoma), unless the potential benefit outweighs the potential risk to the eye.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before Zennux is administered to you:
- if you have ever had an allergic reaction to any muscle relaxant that you have been given during an operation;
- if you have myasthenia gravis, a disease that causes severe muscle weakness, or any other nerve or muscle disease;
- if you are pregnant or have given birth in the last six weeks;
- if you have tetanus, an infection that occurs due to wound contamination;
- if you have tuberculosis or other severe or long-lasting bacterial infections.
- if you have a long-lasting disease that has weakened you.
- if you have a blood disease known as anemia.
- if you are malnourished or unable to absorb nutrients from food (malnutrition).
- if you have liver or kidney problems;
- if you have an autoimmune disease such as a thyroid gland disease (mixedema);
- if you have diseases that cause joint problems (collagen diseases);
- if you have had a blood treatment known as therapeutic plasmapheresis.
- if you have recently had cardiopulmonary bypass.
Children
This medicine is not recommended in children under 12 years of age because the sub-graduation of the pre-filled syringe does not allow for exact administration of the product in this population.
Special care should be taken when this medicine is administered to children over 12 years of age.
Other medicines and Zennux
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
In particular, tell your doctor, pharmacist, or nurse if you are taking or being treated with any of the following:
- medicines used in psychiatry (e.g., phenelzine, promazine);
- medicines against cancer (e.g., cyclophosphamide, thiotepa, irinotecan);
- anesthetic medicines (e.g., ketamine, halothane, enflurane, desflurane, propofol);
- local anesthetic medicines (e.g., lidocaine, procaine, procainamide);
- a medicine used to treat or prevent nausea and vomiting (metoclopramide);
- medicines used for Alzheimer's disease or myasthenia gravis (anticholinesterases such as donepezil, edrophonium, galantamine, neostigmine, pyridostigmine, rivastigmine, and tacrine);
- medicines for the treatment of asthma or other respiratory problems (e.g., bambuterol, terbutalina);
- organic substances containing phosphorus;
- a medicine used to reduce bleeding (aprotinin);
- estrogens and oral contraceptives containing estrogen;
- a medicine to contract the uterus (oxytocin);
- medicines used for inflammatory diseases (steroids such as for rheumatism, etc.);
- medicines used in the treatment of heart rhythm disorders (antiarrhythmic agents such as quinidine, verapamil);
- some antibiotics used to treat bacterial infections (e.g., lincosamides, polymyxins, and aminoglycosides);
- antiepileptic medicines used to stop seizures (e.g., carbamazepine and phenytoin);
- a beta-blocker medicine used to decrease heartbeats (esmolol);
- a medicine to suppress the immune response (azathioprine);
- a medicine used to control overexcitement and/or depression (lithium);
- magnesium salts;
- medicines that increase heart muscle contractions (cardiac glycosides such as digoxin);
- a medicine used to treat high pressure in the eyes, i.e., glaucoma (ecotiopate).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before Zennux is administered to you.
Zennux should only be used during pregnancy if your doctor decides that the benefits to you outweigh any potential risk to the unborn baby.
Caution should be exercised after the administration of suxamethonium to pregnant or postpartum patients.
It is not known if suxamethonium is excreted in breast milk. However, no effect is expected in newborns/breastfed infants, as suxamethonium is rapidly metabolized to an inactive metabolite.
Driving and using machines
It may be dangerous to drive or use machines too soon after receiving this medicine. Your doctor will tell you how long to wait before you can drive or use machines.
Zennux contains sodium.
This medicine contains 27.9 mg of sodium (main ingredient of kitchen/table salt). This is equivalent to 1.4% of the recommended daily intake of sodium for an adult.
3. How Zennux is administered
You will never have to administer this medicine yourself. It will always be administered to you by a qualified healthcare professional.
Your doctor will decide the dose you receive. This depends on your personal needs, body weight, and the amount of muscle relaxation required.
Zennux will be administered to you as an injection into your vein (intravenous use). The pre-filled syringe is not suitable for use in a syringe pump.
If you are given more Zennux than you should
Since this medicine will always be administered under carefully controlled conditions, it is unlikely that you will be given more than you should. In case of overdose, the muscle will remain relaxed for longer than necessary.
If you have any questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common (may affect more than 1 in 10 people):
- visible muscle contractions under the skin
- muscle pain after the operation - your doctor will monitor you to control this.
Common (may affect up to 1 in 10 people)
- allergic reactions: itching, hives, collapse
- increase in the pressure of the fluid in the eye that can cause headache or blurred vision
- increase in stomach pressure
- increase or decrease in your heart rate
- low blood pressure
- proteins in the blood or urine due to muscle damage
- high level of potassium in your blood
- redness of the skin
- rash
Rare (may affect up to 1 in 1,000 people)
- breathing difficulties
- high body temperature.
- difficulty opening the mouth.
Not known (frequency cannot be estimated from the available data)
- swelling (angioedema)
- cardiac arrest
- high or low blood pressure
- excessive saliva production
- excessive mucus production
- temporary loss of air
- muscle damage
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Zennux
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pre-filled syringe, blister, and carton. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store the pre-filled syringe in the closed blister pack until use.
The medicine should be used immediately after opening.
This medicine may be stored for a short period at temperatures not above 25°C. In any case, once it has been taken out of the refrigerator, the medicine should be discarded after 30 days.
Do not use this medicine if you notice visible signs of deterioration.
Any pre-filled syringe, even if partially used, must be disposed of properly after use. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Zennux
- The active substance is suxamethonium chloride.
Each ml of solution for injection contains 10 mg of suxamethonium chloride anhydrous (as 11 mg of suxamethonium chloride dihydrate).
Each pre-filled syringe of 10 ml contains 100 mg of suxamethonium chloride anhydrous (as 110 mg of suxamethonium chloride dihydrate).
- The other ingredients are: sodium chloride, succinic acid, sodium hydroxide or hydrochloric acid (for pH adjustment), water for injections.
Appearance and pack contents
Zennux is a clear, colorless solution for injection in a 10 ml pre-filled syringe, packaged individually in a transparent blister pack. Cartons of 1 or 10 pre-filled syringes.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Laboratoire Aguettant
1 rue Alexander Fleming
69007 Lyon
France
Manufacturer
Laboratoire Aguettant
1, rue Alexander Fleming
69007 LYON
France
Laboratoire Aguettant
Lieu Dit Chantecaille
07340 Champagne
France
Local representative:
Aguettant Ibérica S.L.
Parc Científic de Barcelona
Baldiri Reixac, 4-8 (Torre I)
08028 Barcelona
Date of last revision of this leaflet: December 2024.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gov.es/)
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Instructions for use:
The pre-filled syringe is not suitable for use in a syringe pump.
Please prepare the syringe carefully as follows
The pre-filled syringe is for single patient use only. Discard the syringe after use. Do not reuse the syringe.
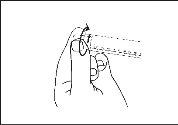
The medicine should be inspected visually for particles and discoloration before administration. Only a clear, colorless solution free of particles or precipitates should be used.
The medicine should not be used if the security seal of the syringe is broken.
The outer surface of the syringe is sterile until the blister is opened. The blister should not be opened until the moment of use.
When handled using an aseptic method, this medicine can be placed on a sterile field once it has been removed from the blister.
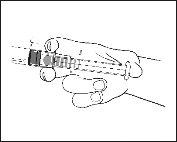
- Remove the pre-filled syringe from the blister.
|
|
|
|
|
|
|
|
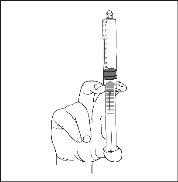
- Connect the syringe to an access device or needle. Push the plunger slowly to inject the required volume.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZENNUX 10 mg/ml injectable solution in pre-filled syringeDosage form: INJECTABLE, 50 mg/mlActive substance: suxamethoniumManufacturer: EthypharmPrescription requiredDosage form: INJECTABLE, 200 U/mlActive substance: botulinum toxinManufacturer: Ipsen PharmaPrescription requiredDosage form: INJECTABLE, 125 Speywood UnitsActive substance: botulinum toxinManufacturer: Ipsen Pharma S.A.U.Prescription required
Online doctors for ZENNUX 10 mg/ml injectable solution in pre-filled syringe
Discuss questions about ZENNUX 10 mg/ml injectable solution in pre-filled syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions