
ZEFFIX 5 mg/ml ORAL SOLUTION


How to use ZEFFIX 5 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Zeffix 5mg/ml Oral Solution
lamivudine
Read all of this leaflet carefully before you start taking this medicine.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Zeffix is and what it is used for
- What you need to know before you take Zeffix
- How to take Zeffix
- Possible side effects
- Storage of Zeffix
- Contents of the pack and other information
1. What Zeffix is and what it is used for
The active substance of Zeffix is lamivudine.
Zeffix is used to treat long-term (chronic) hepatitis B infection in adults.
Zeffix is an antiviral medicine that stops the hepatitis B virus from multiplying and belongs to a group of medicines called nucleoside analogue reverse transcriptase inhibitors (NRTIs).
The hepatitis B virus infects the liver and can cause long-term (chronic) infection, which can lead to liver damage. Zeffix can be used in patients whose liver is damaged but still works normally (compensated liver disease) and in combination with other medicines in patients whose liver is damaged and does not work normally (decompensated liver disease).
Treatment with Zeffix can reduce the amount of hepatitis B virus in your body. This can lead to a reduction in liver damage and an improvement in the functioning of your liver. Not everyone responds to treatment with Zeffix in the same way. Your doctor will monitor the effectiveness of your treatment with regular blood tests.
2. What you need to know before you take Zeffix
Your doctor should offer you advice and testing for HIV infection before you start treatment with lamivudine for hepatitis B infection and during treatment. If you have or get HIV infection, see section 3.
Do not take Zeffix
- if you are allergicto lamivudine or any of the other ingredients of this medicine (listed in section 6).
- Consult your doctorif you think this applies to you.
Warnings and precautions
Some people who take Zeffix or similar medicines have a higher risk of serious side effects. You need to know that there is a higher risk:
- if you have ever had other types of liver disease, such as hepatitis C
- if you are significantly overweight(especially if you are a woman)
- Tell your doctorif you have any of these conditions. You may need extra checks, such as blood tests, while you are taking this medicine. For more information on the risks, see Section 4.
Do not stop taking Zeffixwithout your doctor's advice, as there is a risk that your hepatitis may get worse. When you stop taking Zeffix, your doctor will monitor you for at least four months to check for any problems. This will involve taking blood samples to check for increases in liver enzyme levels that may indicate liver damage. See section 3 for more information on how to take Zeffix.
Protect others
Hepatitis B is spread by having sexual contact with someone who has the disease or by transferring infected blood (for example, by sharing needles). Zeffix does not prevent the risk of passing on hepatitis B infection to others. To prevent others from getting hepatitis B:
- Use a condomwhen you have oral or penetrative sex.
- Avoid the risk of blood transfer— for example, do not share needles.
Other medicines and Zeffix
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those with herbal medicines or medicines without a prescription.
Remember to tell your doctor or pharmacist if you start taking any other medicine while you are taking Zeffix.
These medicines must not be taken with Zeffix:
- medicines, if taken regularly, (usually liquids) that contain sorbitol and other polyalcohols (such as xylitol, mannitol, lactitol, or maltitol)
- other medicines that contain lamivudine, used to treat HIV infection(sometimes also called AIDS virus)
- emtricitabine, used to treat HIV infectionor hepatitis B
- cladribine, used to treat hairy cell leukemia
- Tell your doctorif you are being treated with any of these medicines.
Pregnancy
If you are pregnant, think you may be pregnant, or are planning to have a baby:
- Talk to your doctorabout the risks and benefits of taking Zeffix during pregnancy.
Do not stop taking Zeffix without your doctor's advice.
Breast-feeding
Zeffix can pass into breast milk. If you are breast-feeding or thinking of breast-feeding:
- Talk to your doctorbefore taking Zeffix.
Driving and using machines
Zeffix can make you feel tired, which could affect your ability to drive or use machines.
- Do not drive or use machines unless you are sure it won't affect you.
Zeffix contains sugar, preservatives, propylene glycol, and sodium
If you are diabetic, note that each dose of Zeffix (100 mg – 20 ml) contains 4 g of sugar.
Zeffix contains sucrose. If your doctor has told you that you have an intolerance to some sugars, consult them before taking Zeffix. Sucrose may be harmful to your teeth.
Zeffix contains preservatives (parahydroxybenzoates) that may cause allergic reactions (possibly delayed).
This medicine contains 400 mg of propylene glycol in each 20 ml.
This medicine contains 58.8 mg of sodium (a major component of table/cooking salt) in each 20 ml. This is equivalent to 2.9% of the maximum recommended daily intake of sodium for an adult.
3. How to take Zeffix
Always take this medicine exactly as your doctor has told you.If you are not sure, check with your doctor or pharmacist.
Keep in regular contact with your doctor
Zeffix helps control your hepatitis B infection. You need to keep taking it every day to control the infection and prevent it from getting worse.
- Stay in touch with your doctor and do not stop taking Zeffixwithout your doctor's advice.
How much to take
The usual dose of Zeffix is 20ml(100 mg of lamivudine) once a day.
Your doctor may prescribe a lower dose if you have kidney problems.
- Talk to your doctorif you are in this situation.
Patients who also have or may get HIV infection
If you have or get HIV infection that is not being treated with medicines while you are taking lamivudine for hepatitis B infection, the HIV virus may become resistant to certain HIV medicines and become difficult to treat. Lamivudine is also used to treat HIV infection. Talk to your doctor if you have HIV infection. Your doctor may treat you with another medicine that contains a higher dose of lamivudine, usually 150 mg twice a day, as the lower dose of 100 mg is not enough to treat HIV infection. If you are planning to change your HIV treatment, discuss this change with your doctor before.
- Talk to your doctorif you are in this situation.
Swallow the tablet whole with water. Zeffix can be taken with or without food.
See the diagram and instructions for measuring and taking a dose of medicine after section 6 of this leaflet.
If you take more Zeffix than you should
If you accidentally take too much Zeffix, talk to your doctor or pharmacist, or go to the nearest hospital emergency department for advice. If possible, show them the Zeffix packaging.
If you forget to take Zeffix
If you miss a dose, take it as soon as you remember. Then continue taking it as before. Do not take a double dose to make up for missed doses.
Do not stop taking Zeffix
Do not stop taking Zeffix without talking to your doctor. There is a risk that your hepatitis may get worse (see section 2). When you stop taking Zeffix, your doctor will monitor you for at least four months to check for any problems. This means you will have blood tests to check for increases in liver enzyme levels that may indicate liver damage.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effects reported with Zeffix in clinical trials were tiredness, respiratory tract infections, sore throat, headache, stomach discomfort and pain, nausea, vomiting, and diarrhea, increased liver enzymes, and increased muscle enzymes (see below).
Allergic reaction
This is rare (may affect up to 1 in 1,000 people). The signs include:
- swelling of the eyelids, face, or lips
- difficulty swallowing or breathing.
- Contact a doctor immediatelyif you have these symptoms. Stop taking Zeffix.
Side effects that are thought to be caused by Zeffix:
A very common side effect(may affect more than1 in 10people) that may appear in blood tests is
- an increase in the levels of some liver enzymes (transaminases), which may be a sign of liver inflammation or damage.
A common side effect(may affect up to 1 in 10people) is:
- muscle cramps and pain
- skin rash or "hives" anywhere on the body.
A common side effectthat may appear in blood tests is:
- an increase in the level of a muscle enzyme (creatine phosphokinase) that may be a sign of muscle damage.
A very rare side effect(may affect up to 1 in 10,000 people) is:
- lactic acidosis (excess lactic acid in the blood).
Other side effects
Other side effects have been reported in a small number of people, but their exact frequency is unknown:
- muscle breakdown
- worsening of liver disease if the hepatitis B virus becomes resistant to Zeffix or treatment is stopped. This can be fatal in some people.
A side effect that may appear in blood tests is:
- a decrease in the number of cells involved in blood clotting (thrombocytopenia).
If you get side effects
- Talk to your doctor or pharmacisteven if you think the side effects are not listed in this leaflet.
Reporting side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Zeffix
Keep this medicine out of the sight and reach of children.
Do not take this medicine after the expiry date which is stated on the packaging and bottle.
Do not store above 25°C.
Discard one month after first opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Zeffix contains
The active substance is lamivudine. Each ml of oral solution contains 5 mg of lamivudine.
The other ingredients are: sucrose, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), citric acid, propylene glycol (E1520), sodium citrate, artificial strawberry flavor, artificial banana flavor, purified water.
Appearance and packaging
Zeffix oral solution is available in packs containing a white polyethylene bottle with a child-resistant closure. The solution is clear, colorless, or pale yellow with a strawberry/banana flavor. The bottle contains 240 ml of lamivudine solution (5 mg/ml). The pack includes a graduated oral applicator in ml and an applicator adapter that must be fitted to the bottle before use.
Manufacturer | Marketing Authorization Holder |
GlaxoSmithKline Trading Services Limited 12 Riverwalk, Citywest Business Campus Dublin 24, Ireland D24 YK11 | GlaxoSmithKline Trading Services Limited 12 Riverwalk Citywest Business Campus Dublin 24, Ireland D24 YK11 |
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium GlaxoSmithKline Pharmaceuticals s.a./n.v. Tel: + 32 (0)10 85 52 00 | Lithuania GlaxoSmithKline Trading Services Limited Tel: + 370 80000334 |
| Luxembourg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgium Tel: + 32 (0) 10 85 52 00 |
Czech Republic GlaxoSmithKline s.r.o. Tel: + 420 222 001 111 | Hungary GlaxoSmithKline Trading Services Limited Tel.: + 36 80088309 |
Denmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Trading Services Limited Tel: + 356 80065004 |
Germany GlaxoSmithKline GmbH & Co. KG Tel.: + 49 (0)89 36044 8701 | Netherlands GlaxoSmithKline BV Tel: + 31 (0) 33 2081100 |
Estonia GlaxoSmithKline Trading Services Limited Tel: + 372 8002640 | Norway GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Greece GlaxoSmithKline Μονοπρ?σωπη A.E.B.E. Tel: + 30 210 68 82 100 | Austria GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
Spain GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Poland GSK Services Sp.zo.o. Tel.: + 48 (0)22 576 9000 |
France Laboratoire GlaxoSmithKline Tél.: + 33 (0)1 39 17 84 44 | Portugal Glaxo Wellcome Farmacêutica, Lda. Tel: + 351 21 412 95 00 |
Croatia GlaxoSmithKline Trading Services Limited Tel: + 385 800787089 | Romania GlaxoSmithKline Trading Services Limited Tel: + 40 800672524 |
Ireland GlaxoSmithKline Trading Services Limited Tel: + 353 (0)1 4955000 | Slovenia GlaxoSmithKline Trading Services Limited Tel: + 386 80688869 |
Iceland Vistor hf. Phone: + 354 535 7000 | Slovak Republic GlaxoSmithKline Trading Services Limited Tel: + 421 800500589 |
Italy GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741 111 | Finland GlaxoSmithKline Oy Phone/Tel: + 358 (0)10 30 30 30 |
Cyprus GlaxoSmithKline Trading Services Limited Tel: + 357 80070017 | Sweden GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvia GlaxoSmithKline Trading Services Limited Tel: + 371 80205045 |
Date of last revision of this leaflet:05/2025
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu
How to measure the dose and take the medication
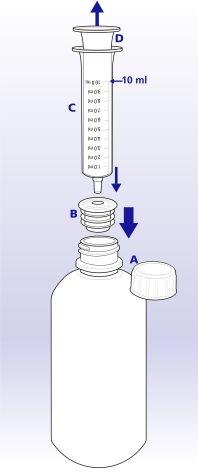
To measure your dose accurately, use the oral applicator included in the packaging (see also Section 3).
When full, the applicator contains 10 ml of solution.
1 Remove the plastic wrapperfrom the applicator/adapter.
- Remove the adapter from the applicator.
- Remove the child-resistant cap (A) from the bottle. Keep the cap.
- Hold the bottle. Insert the plastic adapter (B) into the neck of the bottle as far as it will go.
- Insert the applicator (C) firmly into the adapter.
- Turn the bottle upside down.
- Pull the applicator plunger (D) until the first portion of your full dose is withdrawn.
- Return the bottle to its upright position. Remove the applicator from the adapter.
- Place the applicator in your mouth, with the tip of the applicator towards the inside of your cheek. Slowly press the plunger, allowing time to swallow. Do not press too hard and pour the liquid towards the back of the throat or you may choke.
- Repeat steps 5 to 9 in the same way until you have taken your full dose. For example, if your dose is 20 ml, you need to take 2 complete applicators full of medication.
- Remove the applicator from the bottle and wash it well with clean water. Allow it to dry completely before using it again. Leave the adapter in the bottle.
- Close the bottle tightly with the cap.
Discard the oral solution one month after it was first opened.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZEFFIX 5 mg/ml ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 10 mgActive substance: lamivudineManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: TABLET, 150 mgActive substance: lamivudineManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: TABLET, 150 mgActive substance: lamivudineManufacturer: Viiv Healthcare B.V.Prescription required
Online doctors for ZEFFIX 5 mg/ml ORAL SOLUTION
Discuss questions about ZEFFIX 5 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















