ZARZIO 48 MU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE


How to use ZARZIO 48 MU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Zarzio30MU/0.5ml solution for injection or infusion in a pre-filled syringeZarzio48MU/0.5ml solution for injection or infusion in a pre-filled syringe
filgrastim
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Zarzio and what is it used for
- What you need to know before you use Zarzio
- How to use Zarzio
- Possible side effects
- Storage of Zarzio
- Contents of the pack and further information
- Instructions for use
1. What is Zarzio and what is it used for
Zarzio is a white blood cell growth factor (granulocyte-colony stimulating factor) and belongs to a group of medicines called cytokines. Growth factors are proteins that are produced naturally in the body but can also be made using biotechnology for use as medicines. Zarzio works by stimulating the bone marrow to produce more white blood cells.
A decrease in the number of white blood cells (neutropenia) can occur for various reasons and makes your body less able to fight infections. Zarzio stimulates the bone marrow to produce new white blood cells quickly.
Zarzio can be used:
- to increase the number of white blood cells after chemotherapy to help prevent infections;
- to increase the number of white blood cells after a bone marrow transplant to help prevent infections;
- before receiving high-dose chemotherapy so that the bone marrow produces more stem cells that can be collected and given back to your body after treatment. These can be collected from you or a donor. The stem cells will then return to your bone marrow and produce blood cells;
- to increase the number of white blood cells if you have severe chronic neutropenia to help prevent infections;
- in patients with advanced HIV infection to help reduce the risk of infections.
2. What you need to know before you use Zarzio
Do not use Zarzio
- if you are allergic to filgrastim or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting Zarzio.
Tell your doctor before starting treatment if you have:
- osteoporosis (bone disease);
- sickle cell anaemia as Zarzio may cause sickle cell crisis.
Tell your doctor immediately during treatment with Zarzio, if:
- you experience pain in the upper left side of your abdomen, pain in the left lower side of your chest or pain in the left shoulder tip [these may be symptoms of an enlarged spleen (splenomegaly) or a possible rupture of the spleen].
- you have bleeding or bruising (these may be symptoms of a decrease in the number of platelets in your blood (thrombocytopenia), which can lead to a reduced ability of your blood to clot).
- you experience signs of a sudden allergic reaction, such as rash, itching, or hives on the skin, swelling of the face, lips, tongue, or other parts of the body, difficulty breathing, wheezing, or difficulty swallowing, as these could be signs of a severe allergic reaction (hypersensitivity).
- you experience swelling of the face or ankles, blood in your urine, or if your urine becomes dark or you notice you are urinating less often (glomerulonephritis).
- you experience symptoms of inflammation of the aorta (the large blood vessel that carries blood from the heart to the rest of the body); this has been reported in rare cases in patients with cancer and healthy donors. Symptoms may include fever, abdominal pain, general discomfort, back pain, and increased inflammatory markers. Tell your doctor if you experience these symptoms.
Loss of response to filgrastim
If you lose response to filgrastim treatment or it does not last, your doctor will investigate the reasons, for example, if you have produced antibodies that neutralise the activity of filgrastim.
Your doctor may want to monitor you closely; see section 4 of the leaflet.
If you are a patient with severe chronic neutropenia, you may be at risk of developing blood cancer (leukaemia, myelodysplastic syndrome [MDS]). You should discuss your risk of developing blood cancer and what tests should be done with your doctor. If you develop or are likely to develop blood cancer, you should not use Zarzio unless your doctor tells you to.
If you are a stem cell donor, you must be between 16 and 60 years old.
Be careful with other products that stimulate white blood cells.
Zarzio belongs to a group of medicines that stimulate the production of white blood cells. The healthcare professional treating you should always record in your medical record the exact product you are using.
Other medicines and Zarzio
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding, and fertility
Zarzio has not been tested in pregnant or breastfeeding women.
Zarzio is not recommended during pregnancy.
It is important that you tell your doctor:
- if you are pregnant or breastfeeding,
- if you think you may be pregnant, or
- if you plan to become pregnant.
If you become pregnant during treatment with Zarzio, tell your doctor.
Unless your doctor tells you otherwise, you must stop breastfeeding if you use Zarzio.
Driving and using machines
Zarzio has a minor influence on the ability to drive and use machines. This medicine may cause dizziness. It is recommended that you wait to see how you feel after using Zarzio before driving or using machinery.
Zarzio contains sorbitol and sodium
Zarzio contains sorbitol (E 420).
Sorbitol is a source of fructose. If you (or your child) have hereditary fructose intolerance (HFI), a rare genetic disorder, you must not receive this medicine. Patients with HFI cannot break down fructose, which may cause serious side effects.
Talk to your doctor before receiving this medicine if you (or your child) have HFI or cannot take sweet foods or drinks because they make you feel unwell, with symptoms such as feeling sick, being sick, or having unpleasant effects like bloating, stomach cramps, or diarrhoea.
This medicine contains less than 1 mmol sodium (23 mg) per dose, i.e., it is essentially "sodium-free".
3. How to use Zarzio
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, ask your doctor, nurse, or pharmacist.
How is Zarzio administered and what dose should you use?
Zarzio is usually given as a daily injection under the skin (subcutaneous injection). It can also be given as a daily slow injection into a vein (intravenous infusion). The usual dose depends on your condition and weight. Your doctor will tell you what dose of Zarzio you should use.
Patients who have a bone marrow transplant after chemotherapy:
You will usually receive the first dose of Zarzio at least 24 hours after chemotherapy and at least 24 hours after the bone marrow transplant.
You, or the people looking after you, may be trained on how to give subcutaneous injections so that you can continue treatment at home. However, you should not try to do this until you have been properly trained by a healthcare professional.
For how long will you need to use Zarzio?
You will need to use Zarzio until your white blood cell count is normal. You will have regular blood tests to check the number of white blood cells in your body. Your doctor will tell you for how long you will need to use Zarzio.
Use in children
Zarzio is used to treat children who receive chemotherapy or have a very low white blood cell count (neutropenia). The dose for children receiving chemotherapy is the same as for adults.
Administration of small doses
You should not inject a dose less than 0.3 ml with the pre-filled syringe, as it cannot be accurately measured due to the lack of graduation marks at 0.1 and 0.2 ml.
If necessary, the injection solution can be diluted.
If you use more Zarzio than you should
Do not increase the dose your doctor has prescribed. If you think you have injected more than you should, contact your doctor as soon as possible.
If you forget to use Zarzio
If you miss an injection or the amount injected is too small, contact your doctor as soon as possible. Do not use a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediatelyduring treatment:
- if you have an allergic reaction that includes weakness, low blood pressure, difficulty breathing, swelling of the face (anaphylaxis), skin rash, itchy skin rash (urticaria), swelling of the face, lips, tongue, or throat (angioedema), and difficulty breathing (dyspnoea).
- if you have cough, fever, and difficulty breathing (dyspnoea), as these may be signs of acute respiratory distress syndrome (ARDS).
- if you have pain in the upper left side of your abdomen, pain in the left lower side of your chest, or pain in the left shoulder tip, as these may be signs of an enlarged spleen (splenomegaly) or a possible rupture of the spleen.
- if you are being treated for severe chronic neutropenia and have blood in your urine (haematuria). Your doctor may perform regular urine tests if you have this side effect or if you have protein in your urine (proteinuria).
- if you experience any of the following side effects:
- swelling or inflammation, which may be associated with less frequent urination, difficulty breathing, swelling, and a feeling of fullness in the abdomen, and a general feeling of tiredness. These symptoms usually develop very quickly.
These may be symptoms of a condition called "capillary leak syndrome" and can cause blood to leak from small blood vessels into other parts of your body and may require urgent medical attention.
- if you experience any of the following symptoms:
- fever, or chills, or if you feel very cold, rapid heartbeat, confusion, or disorientation, difficulty breathing, severe pain or discomfort, and sweaty or clammy skin.
These may be symptoms of a condition called "sepsis" (or "septicaemia"), a severe infection in which the body's response to an infection becomes uncontrolled and can be life-threatening and requires urgent medical attention.
- if you experience kidney damage (glomerulonephritis). Kidney damage has been observed in patients treated with filgrastim. Call your doctor immediately if you experience swelling of the face or ankles, blood in your urine, or if your urine becomes dark or you notice you are urinating less often.
A common side effect of filgrastim is muscle or bone pain (musculoskeletal pain), which can be relieved by taking common painkillers. Patients undergoing stem cell or bone marrow transplantation may experience graft-versus-host disease (GVHD). This is a reaction of the donor cells against the patient receiving the transplant, with signs and symptoms including rash on the palms of the hands or soles of the feet and ulcers and sores in the mouth, gut, liver, skin, or eyes, lungs, vagina, and joints. In healthy donors, a very common side effect is an increase in white blood cells in the blood (leucocytosis) and a decrease in platelets, reducing the ability of the blood to clot (thrombocytopenia), both of which will be monitored by your doctor.
Very common side effects(may affect more than 1 in 10 people)
- decrease in the number of platelets, reducing the ability of the blood to clot (thrombocytopenia)
- low red blood cell count (anaemia)
- headache
- diarrhoea
- vomiting
- nausea
- unusual hair loss or thinning (alopecia)
- fatigue
- irritation and swelling of the mucous membranes of the digestive tract (mucositis)
- fever (pyrexia)
Common side effects(may affect up to 1 in 10 people)
- inflammation of the lungs (bronchitis)
- infection of the upper respiratory tract
- urinary tract infection
- decreased appetite
- difficulty sleeping (insomnia)
- dizziness
- loss of sensation, especially in the skin (hypoesthesia)
- tingling or numbness of the hands or feet (paraesthesia)
- low blood pressure (hypotension)
- high blood pressure (hypertension)
- cough
- coughing up blood (haemoptysis)
- pain in the mouth and throat (oropharyngeal pain)
- nosebleeds (epistaxis)
- constipation
- mouth ulcers
- enlargement of the liver (hepatomegaly)
- rash
- redness of the skin (erythema)
- muscle spasms
- pain when urinating (dysuria)
- chest pain
- pain
- general weakness (asthenia)
- general malaise
- swelling of the hands and feet (peripheral oedema)
- increased levels of some enzymes in the blood
- changes in blood biochemical parameters
- transfusion reaction
Uncommon side effects(may affect up to 1 in 100 people)
- increase in white blood cells (leucocytosis)
- allergic reaction (hypersensitivity)
- rejection of the transplanted bone marrow (graft-versus-host disease)
- high levels of uric acid in the blood, which can cause gout (hyperuricaemia) [elevated uric acid in the blood]
- liver damage caused by the blockage of small blood vessels in the liver (veno-occlusive disease)
- abnormal lung function, causing shortness of breath (respiratory failure)
- swelling or fluid in the lungs (pulmonary oedema)
- inflammation of the lungs (interstitial lung disease)
- abnormal chest X-ray (pulmonary infiltration)
- bleeding in the lungs (pulmonary haemorrhage)
- lack of oxygen in the lungs (hypoxia)
- irregular rash (maculopapular rash)
- a disease that causes a decrease in bone density, making them weaker, more fragile, and more prone to breaking (osteoporosis)
- reaction at the injection site
Rare side effects(may affect up to 1 in 1,000 people):
- severe pain in the bones, chest, gut, or joints (sickle cell crisis)
- sudden, potentially life-threatening allergic reaction (anaphylactic reaction)
- joint pain and swelling, similar to gout (pseudogout)
- a change in the way the body regulates fluids, which can cause swelling (fluid volume changes)
- inflammation of the blood vessels in the skin (cutaneous vasculitis)
- painful, dark red ulcers with an inflammatory halo on the limbs and sometimes the face and neck, accompanied by fever (Sweet's syndrome)
- worsening of rheumatoid arthritis
- abnormal urine changes
- decrease in bone density
- inflammation of the aorta (the large blood vessel that carries blood from the heart to the rest of the body), see section 2
- formation of blood cells outside the bone marrow (extramedullary haematopoiesis)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Zarzio
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the syringe after CAD and EXP, respectively. The expiry date is the last day of the month indicated.
Store in a refrigerator (between 2 °C and 8 °C).
Keep the pre-filled syringe in the outer packaging to protect it from light.
Accidental freezing will not cause damage to Zarzio.
The syringe can be taken out of the refrigerator and left at room temperature for a single period of up to 8 days (but at a temperature not above 25 °C). After this period, the product must not be put back in the refrigerator and must be discarded.
Do not use this medicine if you notice discoloration, turbidity, or particles; it should be a clear liquid, colorless to slightly yellowish.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
Zarzio Composition
- The active ingredient is filgrastim.
Zarzio 30 MU/0.5 ml solution for injection or infusion in a pre-filled syringe: each pre-filled syringe contains 30 MU of filgrastim in 0.5 ml, which corresponds to 60 MU/ml.
Zarzio 48 MU/0.5 ml solution for injection or infusion in a pre-filled syringe: each pre-filled syringe contains 48 MU of filgrastim in 0.5 ml, which corresponds to 96 MU/ml.
- The other components are glutamic acid, sorbitol (E 420), polysorbate 80, sodium hydroxide, and water for injections. See section 2 "Zarzio contains sorbitol and sodium".
Product Appearance and Container Contents
Zarzio is a clear and colorless to slightly yellowish solution for injection or infusion, which is supplied in a pre-filled syringe containing 0.5 ml of solution.
Zarzio is available in packs of 1, 3, 5, or 10 pre-filled glass syringes (type I glass) with a plunger stopper (bromobutyl rubber), a stainless steel needle of 29 gauge with an automatic needle protector, and a needle shield (thermoplastic elastomer).
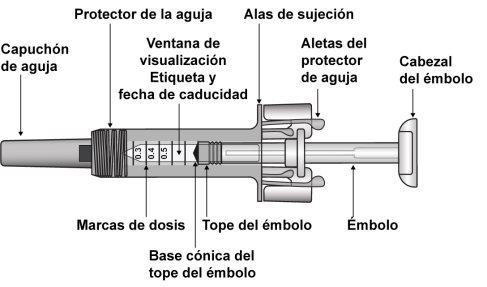
The pre-filled syringe has markings from 0.1 ml to 1 ml; however, it is not designed to measure volumes less than 0.3 ml due to the spring mechanism.
Only some pack sizes may be marketed.
Marketing Authorisation Holder
Sandoz GmbH
Biochemiestr. 10
6250 Kundl
Austria
Manufacturer
Sandoz GmbH
Biochemiestr. 10
6336 Langkampfen
Austria
Novartis Pharmaceutical Manufacturing GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
You can request more information about this medicinal product from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Sandoz nv/sa Tél/Tel: +32 2 722 97 97 | Lietuva Sandoz Pharmaceuticals d.d filialas Tel: +370 5 2636 037 |
| Luxembourg/Luxemburg Sandoz nv/sa (Belgique/Belgien) Tél/Tel.: +32 2 722 97 97 |
Ceská republika Sandoz s.r.o. Tel: +420 234 142 222 | Magyarország Sandoz Hungária Kft. Tel.: +36 1 430 2890 |
Danmark/Norge/Ísland/Sverige Sandoz A/S Tlf/Sími/Tel: +45 63 95 10 00 | Malta Sandoz Pharmaceuticals d.d. Tel: +35699644126 |
Deutschland Hexal AG Tel: +49 8024 908 0 | Nederland Sandoz B.V. Tel: +31 36 52 41 600 |
Eesti Sandoz d.d. Eesti filiaal Tel: +372 665 2400 | Österreich Sandoz GmbH Tel: +43 5338 2000 |
Ελλáδα SANDOZ HELLAS ΜΟΝΟΠΡΟΣΩΠΗ Α.Ε. Τηλ: +30 216 600 5000 | Polska Sandoz Polska Sp. z o.o. Tel.: +48 22 209 70 00 |
España Sandoz Farmacéutica, S.A. Tel: +34 900 456 856 | Portugal Sandoz Farmacêutica Lda. Tel: +351 21 000 86 00 |
France Sandoz SAS Tél: +33 1 49 64 48 00 | România Sandoz Pharmaceuticals SRL Tel: +40 264 50 15 00 |
Hrvatska Sandoz d.o.o. Tel: +385 1 23 53 111 | Slovenija Sandoz farmacevtska družba d.d. Tel: +386 1 580 29 02 |
Ireland Rowex Ltd. Tel: + 353 27 50077 | Slovenská republika Sandoz d.d. - organizačná zložka Tel: +421 2 48 20 0600 |
Italia Sandoz S.p.A. Tel: +39 02 96541 | Suomi/Finland Sandoz A/S Puh/Tel: +358 10 6133 400 |
Κúπρος SANDOZ HELLAS ΜΟΝΟΠΡΟΣΩΠΗ Α.Ε. Τηλ: +30 216 600 5000 | United Kingdom (Northern Ireland) Sandoz GmbH (Austria) Tel: +43 5338 2000 |
Latvija Sandoz d.d. Latvia filiale Tel: +371 67 892 006 |
Date of Last Revision of this Leaflet: {MM/AAAA}
Detailed information on this medicinal product is available on the European Medicines Agency web site: http://www.ema.europa.eu/.
-------------------------------------------------------------------------------------------------------------------------
- Instructions for Use
Follow these instructions to help avoid a possible infection.
It is important that you do not attempt to administer the injection or give it to another person unless your doctor, nurse, or pharmacist has explained how to do it. Read all the instructions before administering an injection. Each sealed blister pack contains a single pre-filled syringe.
Each pre-filled syringe contains 30 MU/0.5 ml or 48 MU/0.5 ml of filgrastim.
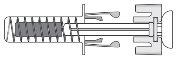
Figure 7-1 Zarzio Pre-filled Syringe with Needle Protector
After the medication has been injected, the needle protector will activate to cover the needle. The needle protector is designed to protect healthcare professionals, caregivers, and patients from accidental needlestick injuries after injection.
Additional Materials Needed for Injection:
gauze
for sharp objects
|
|
Important Safety Information
Caution: Keep the pre-filled syringe out of the reach of children.
- Do not open the outer carton until you are ready to use the pre-filled syringe.
- Do not use the pre-filled syringe if the blister seal is broken, as using it may not be safe for you.
- Do not use the pre-filled syringe if there is liquid in the plastic tray. Do not use the pre-filled syringe if the needle shield is missing or not properly placed. In all these cases, return the complete product packaging to the pharmacy.
- Do not attempt to inject a dose less than 0.3 ml with a pre-filled syringe. It is not possible to accurately measure a dose less than 0.3 ml with the Zarzio pre-filled syringe, as the 0.1 and 0.2 ml gradations are not visible on the syringe cylinder.
- Never leave the pre-filled syringe unattended where others may handle it.
- Do notshake the pre-filled syringe.
- Be careful not to touch the wings of the needle protector before use. If the wings are touched, the needle protector may activate prematurely.
- Do not remove the needle shield until just before administering the injection.
- The pre-filled syringe cannot be reused. Discard the used pre-filled syringe immediately.
- Do not use the syringe if it has been dropped onto a hard surface or after the needle shield has been removed.
Storage of the Zarzio Pre-filled Syringe
- Store the pre-filled syringe in its outer carton to protect it from light. Store it in the refrigerator between 2 °C and 8 °C (36 °F and 46 °F). Do notfreeze.
- Remember to remove the blister pack from the refrigerator and let it reach room temperature for 15-30 minutes before preparing the syringe for injection.
- Do not use the pre-filled syringe after the expiry date stated on the outer carton or on the syringe label. If it has expired, return the complete product packaging to the pharmacy.
- The syringe can be removed from the refrigerator and stored at room temperature for a single period of up to 8 days (but not above 25 °C). At the end of this period, the product cannot be put back in the refrigerator and must be discarded.
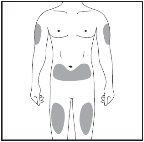
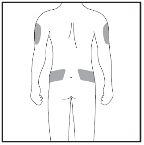
Injection Site
Figure 7-3 Injection Sites
| The injection site is the part of the body where you will use the pre-filled syringe.
|
|
|
Preparing the Zarzio Pre-filled Syringe for Use
- Remove the blister pack containing the pre-filled syringe from the refrigerator and let it unopenedfor approximately 15-30 minutes to reach room temperature.
- When you are ready to use the pre-filled syringe, open the blister pack and wash your hands well with soap and water.
- Clean the injection site with an alcohol swab.
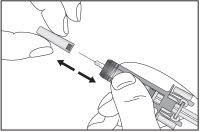
- Remove the pre-filled syringe from the blister pack by holding it by the middle, as shown in Figure 7-4. Do not grasp the plunger rod or the needle shield.
Figure 7-4 Remove the Pre-filled Syringe from the Blister Pack
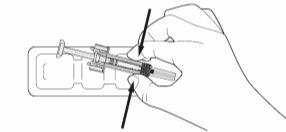
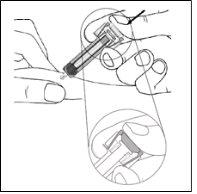
- Check that the transparent plastic needle protector is situated over the glass syringe cylinder. If the transparent needle protector is covering the needle shield (as shown in Figure 7-5), the syringe has been activated; DO NOT USEthis syringe and take a new one. Figure 7-6 shows a syringe ready for use.
Figure 7-5 DO NOT USE
| In this configuration, the needle protector is ACTIVATED: DO NOT USEthe pre-filled syringe |
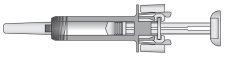
Figure 7-6 Ready for Use
| In this configuration, the needle protector is NOT ACTIVATEDand the syringe is ready for use. |
- Inspect the pre-filled syringe. The liquid should be clear. The color may be colorless to slightly yellowish. DO NOT USEthe pre-filled syringe if you notice particles or color changes; return the pre-filled syringe and its packaging to the pharmacy.
- DO NOT USEthe pre-filled syringe if it is broken or the needle protector has been activated. In all these cases, return the complete product packaging to the pharmacy.
How to Use the Pre-filled Syringe
Figure 7-7 Remove the Needle Shield
| Gently pull off the needle shield from the pre-filled syringe. Discard the needle shield. You may see a drop of liquid at the tip of the needle. This is normal. |
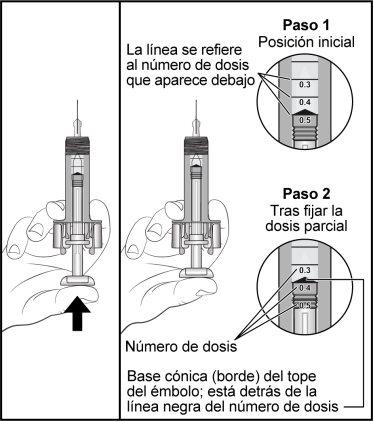
Hold the syringe as shown in the figure, slowly press the plunger to expel excess medication until the edge of the conical base of the plunger is aligned with the syringe marking corresponding to the prescribed dose. The following example is for a dose of 0.4 ml. Be careful not to touch the wings of the needle protector before using it. The needle protector may activate prematurely. Check again that the pre-filled syringe has the correct dose of Zarzio. Call your doctor or nurse if you have problems measuring or injecting the dose of Zarzio. | |
Figure 7-8 Example for a 0.4 ml Dose
| |
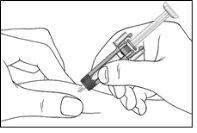
Figure 7-9 Insert the Needle
| Gently pinch the skin at the injection site and insert the needle as shown in the figure. Push the needle all the way in to ensure that all the medication is administered. |
Figure 7-10 Press the Plunger
| Holding the pre-filled syringe as shown, slowly press the plunger until it reaches the stop, so that the plunger head is completely between the wings of the needle protector. Keep the plunger fully pressed while holding the syringe in place for 5 seconds. |
Figure 7-11 Remove the Needle
| Keep the plunger fully pressedwhile carefully pulling out the needle from the injection site. |
Figure 7-12 Release the Plunger
| Release the plunger slowly and let the needle protector automatically cover the exposed needle. You may see a small amount of blood at the injection site. You can apply gentle pressure with a cotton ball or gauze to the injection site for 10 seconds. Do not rub the injection site. If necessary, you can apply an adhesive plaster. |
Disposal Instructions
Figure 7-13 Disposal
| Discard the used syringe in a puncture-resistant container (a container with a tight-fitting lid). For your health and safety, and that of others, used needles and syringes must neverbe reused. |
-------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The solution should be inspected visually before use. Only clear solutions without particles should be used. Accidental exposure to freezing temperatures does not adversely affect the stability of Zarzio.
Zarzio does not contain preservatives: in view of the possible risk of microbiological contamination, Zarzio syringes are for single use only.
Prior dilution for administration (optional)
Zarzio can be diluted, if necessary, in a glucose solution of 50 mg/ml (5%). Zarzio must not be diluted with sodium chloride solutions.
It is not recommended to dilute to final concentrations < 0.2 MU/ml (2 micrograms/ml) under any circumstances.
In patients treated with filgrastim diluted to concentrations < 1.5 MU/ml (15 micrograms/ml), human serum albumin (HSA) must be added to a final concentration of 2 mg/ml.
Example: if the final injection volume is 20 ml and the total dose of filgrastim is less than 30 MU (300 micrograms), 0.2 ml of a 200 mg/ml (20%) human serum albumin solution Ph. Eur. must be added.
Diluted in a glucose solution of 50 mg/ml (5%), filgrastim is compatible with glass and various plastics, including polyvinyl chloride, polyolefin (a copolymer of polypropylene and polyethylene), and polypropylene.
After dilution: it has been demonstrated that, during use, the diluted solution for infusion remains physically and chemically stable for 24 hours at 2 – 8 °C. From a microbiological point of view, the product must be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user and normally should not exceed 24 hours at 2 – 8 °C, unless the dilution has been performed under validated and controlled aseptic conditions.
Use of the pre-filled syringe with needle protector
The needle protector covers the needle after injection to prevent accidental needlestick injuries. This does not affect the way the syringe is used. Push the plunger slowly and evenly until the entire dose has been administered.
Dose quality and the plunger cannot advance further. Remove the syringe while maintaining pressure on the plunger. The safety shield for the needle will cover it once the plunger is released.
Elimination
The elimination of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZARZIO 48 MU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 12 IU/0.2 mlActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.3 mg / prefilled syringeActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.3 mg / prefilled syringeActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for ZARZIO 48 MU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about ZARZIO 48 MU/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions