XTANDI 40 mg SOFT CAPSULES

How to use XTANDI 40 mg SOFT CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
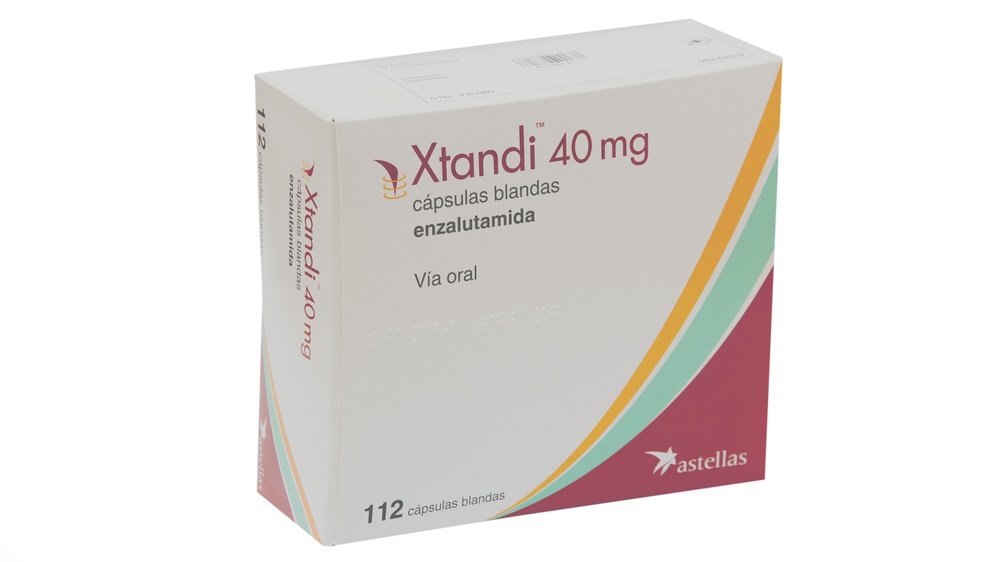
Xtandi 40mg Soft Capsules
enzalutamide
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, even if you think the side effects are not serious, or if they are listed in section 4.
Contents of the pack
- What is Xtandi and what is it used for
- What you need to know before you take Xtandi
- How to take Xtandi
- Possible side effects
- Storage of Xtandi
- Contents of the pack and other information
1. What is Xtandi and what is it used for
Xtandi contains the active substance enzalutamide. Xtandi is used to treat adult men with prostate cancer:
- That has stopped responding to hormone therapy or surgical treatment to reduce testosterone
Or
- That has spread to other parts of the body and responds to hormone therapy or surgical treatment to reduce testosterone
Or
- That has undergone previous prostate removal or radiation and has a rapid increase in PSA, but the cancer has not spread to other parts of the body and responds to hormone therapy to reduce testosterone
How Xtandi works
Xtandi is a medicine that works by blocking the activity of certain hormones called androgens (such as testosterone). By blocking androgens, enzalutamide causes prostate cancer cells to stop growing and dividing.
2. What you need to know before you take Xtandi
Do not take Xtandi
- If you are allergic to enzalutamide or any of the other ingredients of this medicine (listed in section 6)
- If you are pregnant or may become pregnant (see “Pregnancy, breast-feeding and fertility”)
Warnings and precautions
Seizures
Seizures have been reported in 6 out of 1,000 people taking Xtandi, and in less than 3 out of 1,000 people taking placebo (see “Other medicines and Xtandi” below and “Possible side effects” in section 4).
If you are taking a medicine that may cause seizures or may increase the risk of having seizures (see “Other medicines and Xtandi” below).
If you have a seizure during treatment:
Talk to your doctor as soon as possible. Your doctor may decide that you should stop taking Xtandi.
Posterior Reversible Encephalopathy Syndrome (PRES)
Rare cases of PRES, a rare, reversible disease that affects the brain, have been reported in patients treated with Xtandi. If you have a seizure, worsening headache, confusion, blindness, or other vision problems, contact your doctor as soon as possible. (See also section 4 “Possible side effects”).
Risk of new cancers (secondary primary malignancies)
There have been reports of new (second) cancers in patients treated with Xtandi, including bladder and colon cancer.
Talk to your doctor as soon as possible if you notice any signs of gastrointestinal bleeding, blood in your urine, or frequently feel an urgent need to urinate while taking Xtandi.
Difficulty swallowing related to the formulation of the product
Cases of patients who have experienced difficulty swallowing this medicine, including cases of choking, have been reported. Difficulty swallowing and choking episodes were more frequently observed in patients receiving capsules, which may be related to the larger size of the product. Swallow the capsules whole with a sufficient amount of water.
If you have difficulty swallowing large capsules or a history of dysphagia, you may have difficulty swallowing Xtandi capsules or be at risk of choking. An alternative could be to take Xtandi in tablets, consult your doctor.
Talk to your doctor before starting Xtandi
- If you have ever developed severe skin rash, skin peeling, and/or mouth ulcers after taking Xtandi or other medicines
- If you are taking medicines to prevent blood clots (e.g., warfarin, acenocoumarol, clopidogrel)
- If you are receiving chemotherapy, such as docetaxel
- If you have liver problems
- If you have kidney problems
Tell your doctor if you have:
Any heart or blood vessel problems, including heart rhythm problems (arrhythmias), or if you are being treated with medicines to correct these problems. The risk of heart rhythm problems may be increased with the use of Xtandi.
If you are allergic to enzalutamide, its administration could cause a rash or swelling of the face, lips, tongue, or throat. If you are allergic to enzalutamide or any of the other ingredients of this medicine, do not take Xtandi.
Severe skin rash, skin peeling, and/or mouth ulcers, including Stevens-Johnson syndrome, have been reported in association with Xtandi treatment. Stop using Xtandi and seek medical attention immediately if you notice any of the symptoms related to these severe skin reactions described in section 4.
If any of the above applies to you or if you are not sure, talk to your doctor before taking this medicine.
Children and adolescents
This medicine must not be given to children or adolescents.
Other medicines and Xtandi
Tell your doctor if you are taking, have recently taken, or might take any other medicines. You need to know the names of the medicines you are taking. Carry a list of them with you to show your doctor when you are prescribed a new medicine. Do not start or stop taking any medicine before talking to the doctor who prescribed Xtandi.
Tell your doctor if you are taking any of the following medicines. These medicines may increase the risk of having a seizure when taken with Xtandi:
- Certain medicines used to treat asthma and other respiratory diseases (e.g., aminophylline, theophylline).
- Medicines used to treat certain psychiatric disorders, such as depression and schizophrenia (e.g., clozapine, olanzapine, risperidone, ziprasidone, bupropion, lithium, chlorpromazine, mesoridazine, thioridazine, amitriptyline, desipramine, doxepin, imipramine, maprotiline, mirtazapine).
- Certain pain medicines (e.g., pethidine).
Tell your doctor if you are taking any of the following medicines. These medicines may affect the effect of Xtandi or Xtandi may affect the effect of these medicines.
This includes certain medicines used for:
- Lowering cholesterol (e.g., gemfibrozil, atorvastatin, simvastatin)
- Treating pain (e.g., fentanyl, tramadol)
- Treating cancer (e.g., cabazitaxel)
- Treating epilepsy (e.g., carbamazepine, clonazepam, phenytoin, primidone, valproic acid)
- Treating certain psychiatric disorders such as severe anxiety or schizophrenia (e.g., diazepam, midazolam, haloperidol)
- Treating sleep disorders (e.g., zolpidem)
- Treating heart problems or lowering blood pressure (e.g., bisoprolol, digoxin, diltiazem, felodipine, nicardipine, nifedipine, propranolol, verapamil)
- Treating severe inflammatory diseases (e.g., dexamethasone, prednisolone)
- Treating HIV infection (e.g., indinavir, ritonavir)
- Treating bacterial infections (e.g., clarithromycin, doxycycline)
- Treating thyroid disorders (e.g., levothyroxine)
- Treating gout (e.g., colchicine)
- Treating stomach disorders (e.g., omeprazole)
- Preventing heart problems or stroke (e.g., dabigatran etexilate)
- Preventing organ rejection (e.g., tacrolimus)
Xtandi may interfere with some medicines used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with certain medicines (e.g., methadone [used for pain relief and as part of drug detoxification], moxifloxacin [an antibiotic], antipsychotics [used for severe mental illnesses]).
Tell your doctor if you are taking any of the above medicines. It may be necessary to adjust the dose of Xtandi or any other medicine you are taking.
Pregnancy, breast-feeding and fertility
- Xtandi is not indicated in women. This medicine may harm the fetus or cause an abortion if taken by a pregnant woman. It must not be given to pregnant women, women who may become pregnant, or women who are breast-feeding.
- This medicine may affect male fertility.
- If you have sex with a woman of childbearing age, you must use a condom and another effective contraceptive method during treatment and for 3 months after treatment with this medicine. If you have sex with a pregnant woman, you must use a condom to protect the fetus.
- In the case of female caregivers, see section 3 “How to take Xtandi” for handling and administration recommendations.
Driving and using machines
Xtandi may have a moderate influence on the ability to drive and use machines. Seizures have been reported in patients who have taken Xtandi. If you are at a higher risk of having seizures, you should talk to your doctor.
Xtandi contains sorbitol
This medicine contains 57.8 mg of sorbitol (a type of sugar) in each soft capsule.
3. How to take Xtandi
Follow exactly the instructions of administration of this medicine given to you by your doctor. If you are not sure, talk to your doctor.
The recommended dose is 160 mg (four soft capsules), taken at the same time once a day.
How to take Xtandi
- Swallow the soft capsules whole with a sufficient amount of water.
- Do not chew, dissolve, or open the soft capsules before swallowing.
- Xtandi can be taken with or without food.
- Xtandi should not be handled by anyone other than the patient or their caregivers. Pregnant women or women who may become pregnant should not handle Xtandi capsules without protection (e.g., gloves).
It is also possible that your doctor will prescribe other medicines while you are taking Xtandi.
If you take more Xtandi than you should
If you take more capsules than prescribed, stop taking Xtandi and contact your doctor. You may have a higher risk of having a seizure or other side effects.
If you forget to take Xtandi
- If you forget to take Xtandi at the usual time, take the usual dose as soon as you remember.
- If you forget to take Xtandi for the whole day, take the usual dose the next day.
- If you forget to take Xtandi for more than one day, talk to your doctor immediately.
- Do not take a double doseto make up for forgotten doses.
If you stop taking Xtandi
Do not stop taking this medicine unless your doctor tells you to.
If you have difficulty swallowing large capsules or a history of dysphagia
Xtandi capsules should not be given to patients with difficulty swallowing large capsules or patients with dysphagia. Instead, enzalutamide in tablets is recommended.
If you have difficulty swallowing large capsules or a history of dysphagia, you may have difficulty swallowing Xtandi capsules or be at risk of choking. An alternative could be to take Xtandi in tablets, consult your doctor.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seizures
Seizures have been reported in 6 out of 1,000 people taking Xtandi, and in less than 3 out of 1,000 people taking placebo.
Seizures are more likely if you take a higher dose of this medicine than recommended, if you take certain medicines, or if you have a higher than usual risk of having a seizure.
If you have a seizure, talk to your doctor as soon as possible. Your doctor may decide that you should stop taking Xtandi.
Posterior Reversible Encephalopathy Syndrome (PRES)
Rare cases of PRES (affecting up to 1 in 1,000 people), a rare, reversible disease that affects the brain, have been reported in patients treated with Xtandi. If you have a seizure, worsening headache, confusion, blindness, or other vision problems, contact your doctor as soon as possible.
Other possible side effects are:
Very common(may affect more than 1 in 10 people)
Fatigue, falls, bone fractures, hot flushes, high blood pressure
Common(may affect up to 1 in 10 people)
Headache, feeling anxious, dry skin, itching, difficulty remembering, blockage of heart arteries (ischemic heart disease), increase in breast size in men (gynecomastia), nipple pain, breast tenderness, restless legs syndrome, difficulty concentrating, memory loss, change in taste, difficulty thinking clearly
Uncommon(may affect up to 1 in 100 people)
Hallucinations, low white blood cell count, increased liver enzyme levels in blood tests (a sign of liver problems)
Frequency not known(frequency cannot be estimated from the available data)
Muscle pain, muscle spasms, muscle weakness, back pain, changes in electrocardiogram (QT interval prolongation), difficulty swallowing this medicine including choking, stomach upset including feeling sick (nausea), a skin reaction that causes red spots or patches on the skin, which may look like a target or a “bull’s eye” with a red center surrounded by pale red rings (erythema multiforme) or other severe skin reaction that presents with red patches, not raised, target-like or circular on the trunk, often with central blisters, skin peeling, mouth ulcers, throat ulcers, nose ulcers, genital ulcers, and eye ulcers that may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome), rash, vomiting, swelling of the face, lips, tongue, and/or throat, decrease in blood platelet count (which may increase the risk of bleeding or bruising), diarrhea, decreased appetite
Reporting of side effects
If you experience any side effects, talk to your doctor, even if you think the side effects are not serious, or if they are listed in section 4. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Xtandi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the blister pack after “EXP”. The expiry date is the last day of the month shown.
No special storage conditions are required.
Do not take any capsule that is damaged or shows signs of tampering.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
Xtandi composition
- The active ingredient is enzalutamide. Each soft capsule contains 40 mg of enzalutamide.
- The other components of the soft capsule are macrogol-8 caprylocapric glycerides, butylhydroxyanisole (E320), and butylhydroxytoluene (E321).
- The components of the soft capsule shell are gelatin, sorbitol sorbitan solution (see section 2), glycerol, titanium dioxide (E171), and purified water.
- The ink components are black iron oxide (E172) and polyvinyl acetate phthalate.
Product appearance and container contents
- Xtandi soft capsules are oblong soft capsules (approximately 20 mm x 9 mm), white to off-white in color, with "ENZ" printed on one face.
- Each container contains 112 soft capsules in 4 blisters in pocket packs of 28 soft capsules each.
Marketing authorization holder
Astellas Pharma Europe B.V.
Sylviusweg 62
2333 BE Leiden
Netherlands
Manufacturer
Delpharm Meppel B.V.
Hogemaat 2
7942 JG Meppel
Netherlands
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Astellas Pharma B.V. Branch Tel: + 32 (0)2 5580710 | Lithuania Astellas Pharma d.o.o. Tel: + 370 37 408 681 |
| Luxembourg Astellas Pharma B.V. Branch Belgium Tel: + 32 (0)2 5580710 |
Czech Republic Astellas Pharma s.r.o. Tel: + 420 221 401 500 | Hungary Astellas Pharma Kft. Tel: + 36 1 577 8200 |
Denmark Astellas Pharma a/s Tel: + 45 43 430355 | Malta Astellas Pharmaceuticals AEBE Tel: + 30 210 8189900 |
Germany Astellas Pharma GmbH Tel: + 49 (0)89 454401 | Netherlands Astellas Pharma B.V. Tel: + 31 (0)71 5455745 |
Estonia Astellas Pharma d.o.o. Tel: + 372 6 056 014 | Norway Astellas Pharma Tel: + 47 66 76 46 00 |
Astellas Pharmaceuticals AEBE Tel: + 30 210 8189900 | Austria Astellas Pharma Ges.m.b.H. Tel: + 43 (0)1 8772668 |
Spain Astellas Pharma S.A. Tel: + 34 91 4952700 | Poland Astellas Pharma Sp.z.o.o. Tel: + 48 225451 111 |
France Astellas Pharma S.A.S. Tel: + 33 (0)1 55917500 | Portugal Astellas Farma, Lda. Tel: + 351 21 4401300 |
Croatia Astellas d.o.o. Tel: + 385 1 670 01 02 | Romania S.C. Astellas Pharma SRL Tel: + 40 (0)21 361 04 95 |
Ireland Astellas Pharma Co. Ltd. Tel: + 353 (0)1 4671555 | Slovenia Astellas Pharma d.o.o. Tel: + 386 14011 400 |
Iceland Vistor Tel: + 354 535 7000 | Slovak Republic Astellas Pharma s.r.o. Tel: + 421 2 4444 2157 |
Italy Astellas Pharma S.p.A. Tel: + 39 (0)2 921381 | Finland Astellas Pharma Tel: + 358 (0)9 85606000 |
Tel: + 30 210 8189900 | Sweden Astellas Pharma AB Tel: + 46 (0)40‑650 15 00 |
Latvia Astellas Pharma d.o.o. Tel: + 371 67 619365 |
Date of last revision of this leaflet:MM/AAAA.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to XTANDI 40 mg SOFT CAPSULESDosage form: CAPSULE, 40 mgActive substance: enzalutamideManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 40 mgActive substance: enzalutamideManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: TABLET, 80 mgActive substance: enzalutamideManufacturer: Sandoz Farmaceutica S.A.Prescription required
Online doctors for XTANDI 40 mg SOFT CAPSULES
Discuss questions about XTANDI 40 mg SOFT CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions