
XELJANZ 1 mg/mL ORAL SOLUTION


How to use XELJANZ 1 mg/mL ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
XELJANZ 1 mg/ml Oral Solution
tofacitinib
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
In addition to this leaflet, your doctor will also give you a patient information card, which contains important safety information that you need to know before taking XELJANZ and during treatment with XELJANZ. Keep this patient information card with you.
Contents of the Package Leaflet
- What is XELJANZ and what is it used for
- What you need to know before you take XELJANZ
- How to take XELJANZ
- Possible side effects
- Storage of XELJANZ
- Contents of the pack and further information
- Instructions for use of XELJANZ oral solution
1. What is XELJANZ and what is it used for
XELJANZ 1 mg/ml oral solution is a medicine that contains the active substance tofacitinib.
XELJANZ 1 mg/ml oral solution is used to treat active polyarticular juvenile idiopathic arthritis, a long-term disease that mainly causes pain and inflammation of the joints in patients aged 2 years and older.
XELJANZ 1 mg/ml oral solution is also used to treat juvenile psoriatic arthritis, a condition that is an inflammatory disease of the joints, often accompanied by psoriasis, in patients aged 2 years and older.
XELJANZ 1 mg/ml oral solution can be used in combination with methotrexate when previous treatment for polyarticular juvenile idiopathic arthritis or juvenile psoriatic arthritis has not been effective or has not been well tolerated. XELJANZ 1 mg/ml oral solution can also be taken as the only medicine in cases where treatment with methotrexate is not tolerated or is not recommended.
2. What you need to know before you take XELJANZ
Do not take XELJANZ
- if you are allergic to tofacitinib or any of the other ingredients of this medicine
- (listed in section 6)
- if you have a severe infection such as blood infection or active tuberculosis
- if you have been told you have severe liver problems, such as cirrhosis (scarring of the liver)
- if you are pregnant or breastfeeding
Please contact your doctor if you have any doubts about any of the above points.
Warnings and precautions
Talk to your doctor or pharmacist before taking XELJANZ:
- if you think you have an infection or have symptoms of an infection such as fever, sweating, chills, muscle pain, cough, difficulty breathing, phlegm or changes in phlegm, weight loss, hot, red or painful skin or sores on the body, difficulty or pain when swallowing, diarrhea or stomach pain, burning when urinating or urinating more frequently than usual, or if you feel very tired
- if you have any disease that increases the likelihood of infection (e.g. diabetes, HIV/AIDS or a weakened immune system)
- if you have any type of infection, are receiving treatment for any infection, or if you have infections that keep coming back. Inform your doctor immediately if you do not feel well. XELJANZ may reduce the body's ability to respond to infections and may worsen an existing infection or increase the likelihood of contracting a new infection
- if you have or have had tuberculosis or have been in close contact with someone with tuberculosis. Your doctor will perform a tuberculosis test before starting treatment with XELJANZ and may repeat the test during treatment
- if you have any chronic lung disease
- if you have liver problems
- if you have or have had hepatitis B or hepatitis C (viruses that affect the liver). The virus may become active while taking XELJANZ. Your doctor may perform blood tests for hepatitis before starting treatment with XELJANZ and while taking XELJANZ
- if you have ever had any type of cancer, and also if you currently smoke or have smoked in the past. XELJANZ may increase the risk of certain types of cancer. Leukocyte cancer, lung cancer, and other types of cancer (such as breast, skin, prostate, and pancreatic cancer) have been reported in patients treated with XELJANZ. If you develop cancer while taking XELJANZ, your doctor will assess whether to interrupt treatment with XELJANZ
- if you have a known risk of fractures, for example, if you are over 65 years old, are female, or take corticosteroids (e.g. prednisone)
- if you have a high risk of developing skin cancer, your doctor may recommend periodic skin checks while taking XELJANZ
- if you have had diverticulitis (a type of inflammation of the large intestine) or stomach or intestinal ulcers (see section 4)
- if you have kidney problems
- if you plan to get vaccinated, inform your doctor. Certain types of vaccines should not be given when taking XELJANZ. Before starting XELJANZ, you should be up to date with all recommended vaccines. Your doctor will decide if you need to get vaccinated against shingles
- if you have heart problems, high blood pressure, high cholesterol, and also if you currently smoke or have smoked in the past.
There have been reports of patients treated with XELJANZ who have developed blood clots in the lungs or veins. Your doctor will review your risk of developing blood clots in the lungs or veins and determine if XELJANZ is suitable for you. If you have already had problems with developing blood clots in the lungs and veins or are at higher risk of developing them [e.g. if you are significantly overweight, have cancer, heart problems, diabetes, have had a heart attack (in the last 3 months), have had recent major surgery, if you use hormonal contraceptives/hormone replacement therapy, if you have been identified with any coagulation disorder in you or your close relatives], if you currently smoke or have smoked in the past, your doctor may decide that XELJANZ is not suitable for you.
Talk to your doctor immediately if you experience sudden shortness of breath or difficulty breathing, chest pain or pain in the upper back, swelling in the legs or arms, pain or tenderness on touch in the legs, or redness or color change in the legs or arms while taking XELJANZ, as these may be signs of a blood clot in the lungs or veins.
Talk to your doctor immediately if you experience severe changes in vision (blurred vision, partial or total loss of vision), as this may be a sign of blood clots in the eyes.
There have been reports of patients treated with XELJANZ who have had a heart problem, including a heart attack. Your doctor will assess your risk of developing a heart problem and determine if XELJANZ is suitable for you. Talk to your doctor immediately if you experience signs and symptoms of a heart attack, such as severe chest pain or pressure (which may spread to the arms, jaw, neck, back), difficulty breathing, cold sweat, dizziness or sudden dizziness.
Additional monitoring tests
Your doctor should perform blood tests before you start taking XELJANZ, after 4 to 8 weeks of treatment, and then every 3 months, to determine if you have a low count of white blood cells (neutrophils or lymphocytes) or a low count of red blood cells (anemia).
Do not take XELJANZ if your count of white blood cells (neutrophils or lymphocytes) or your count of red blood cells is too low. If necessary, your doctor may suspend your treatment with XELJANZ to reduce the risk of infection (white blood cell count) or anemia (red blood cell count).
Your doctor may also perform other tests, for example, to check cholesterol levels in the blood or to monitor the condition of your liver. Your doctor should evaluate your cholesterol levels within 8 weeks of starting treatment with XELJANZ. Your doctor should perform liver tests periodically.
Elderly patients
The safety and efficacy of tofacitinib 1 mg/ml oral solution have not been established in elderly patients.
Asian patients
A higher number of shingles cases have been observed in Japanese and Korean patients. Inform your doctor if you notice painful blisters on the skin.
You may also have a higher risk of developing certain lung problems. Inform your doctor if you notice any difficulty breathing.
Children and adolescents
This medicine should not be given to patients under 2 years of age.
This medicine contains propylene glycol and should be used with caution in patients aged 2 years and older and only if recommended by the doctor (see "XELJANZ contains propylene glycol").
Other medicines and XELJANZ
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor if you have diabetes or are taking medicines to treat diabetes. Your doctor may decide that you need less diabetes medicine while taking tofacitinib.
Some medicines should not be taken with XELJANZ. If they are taken with XELJANZ, they may change the level of XELJANZ in your body, and the dose of XELJANZ may need to be adjusted. Tell your doctor if you are using medicines that contain any of the following active substances:
- antibiotics such as rifampicin, used to treat bacterial infections
- fluconazole, ketoconazole, used to treat fungal infections
The use of XELJANZ with medicines that suppress the immune system, including targeted biological therapies (antibodies), such as those that inhibit tumor necrosis factor, interleukin-17, interleukin-12/interleukin-23, integrin antagonists, and strong chemical immunosuppressants, including azathioprine, mercaptopurine, cyclosporine, and tacrolimus, is not recommended. The use of XELJANZ with these medicines may increase the risk of side effects, including infection.
Severe infections and fractures may occur more frequently in people who also take corticosteroids (e.g. prednisone).
Pregnancy and breastfeeding
If you are a woman of childbearing age, you must use effective contraception during treatment with XELJANZ and for at least 4 weeks after the last dose.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. XELJANZ should not be used during pregnancy. Inform your doctor immediately if you become pregnant while taking XELJANZ.
If you are taking XELJANZ and are breastfeeding, stop breastfeeding until you talk to your doctor about stopping treatment with XELJANZ.
Driving and using machines
XELJANZ has no or negligible influence on your ability to drive or use machines.
XELJANZ contains propylene glycol
This medicine contains 2.39 mg of propylene glycol in each ml of oral solution.
XELJANZ contains sodium benzoate
This medicine contains 0.9 mg of sodium benzoate in each ml of oral solution.
XELJANZ contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per ml; i.e. it is essentially "sodium-free".
3. How to take XELJANZ
This medicine has been prescribed and supervised by a specialist doctor who knows how to treat your disease.
Follow the instructions for administration of this medicine exactly as indicated by your doctor, do not exceed the recommended dose. If in doubt, consult your doctor or pharmacist again.
The recommended dose in patients from 2 years of age is based on the following classification by weight (see Table 1):
Table 1: XELJANZ dosing for patients with polyarticular juvenile idiopathic arthritis and juvenile psoriatic arthritis from 2 years of age:
Body weight (kg) | Dosing regimen |
10 - < 20 | 3.2 mg (3.2 ml of oral solution) twice a day |
20 - < 40 | 4 mg (4 ml of oral solution) twice a day |
≥ 40 | 5 mg (5 ml of oral solution or 5 mg film-coated tablets) twice a day |
Your doctor may reduce the dose if you have liver or kidney problems, or if you are prescribed certain medicines. Your doctor may also interrupt treatment temporarily or permanently if blood tests show low counts of white blood cells or red blood cells.
If you have polyarticular juvenile idiopathic arthritis or juvenile psoriatic arthritis, your doctor may change your treatment from XELJANZ 5 ml oral solution twice a day to XELJANZ 5 mg film-coated tablets twice a day.
XELJANZ is for oral use. You can take XELJANZ with or without food.
Try to take XELJANZ at the same time every day (in the morning and in the evening).
If you take more XELJANZ than you should
If you take more XELJANZ 1 mg/ml oral solution than you should, inform your doctor or pharmacist immediately.
If you forget to take XELJANZ
Do not take a double dose to make up for forgotten doses. Take the next dose at the usual time and continue as before.
If you stop taking XELJANZ
Do not stop taking XELJANZ without talking to your doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Some may be serious and require medical attention.
Adverse effects in patients with polyarticular juvenile idiopathic arthritis and juvenile psoriatic arthritis were consistent with those observed in adult patients with rheumatoid arthritis, with the exception of some infections (flu, pharyngitis, sinusitis, viral infection) and gastrointestinal or general disorders (abdominal pain, nausea, vomiting, fever, headache, cough), which were more frequent in the pediatric population with juvenile idiopathic arthritis.
Possible Serious Adverse Effects
In rare cases, infections can be fatal. Cases of lung cancer, white blood cell cancer, and myocardial infarction have also been reported.
If you notice any of the following serious adverse effects, inform your doctor immediately.
Signs of severe infection (frequent) include
- fever and chills
- cough
- blisters on the skin
- stomach pain
- persistent headache
Signs of ulcers or holes (perforations) in the stomach (infrequent) include
- fever
- stomach pain or abdominal pain
- blood in stool
- unjustified changes in bowel habits
Stomach ulcers or intestinal ulcers occur more frequently in patients who are also being treated with non-steroidal anti-inflammatory drugs or corticosteroids (e.g., prednisone).
Signs of allergic reactions (unknown frequency) include
- chest tightness
- wheezing
- severe dizziness or vertigo
- swelling of the lips, tongue, or throat
- hives (itching and hives)
Signs of blood clots in the lungs, veins, or eyes (infrequent: venous thromboembolism) include
- sudden shortness of breath or difficulty breathing
- chest pain or upper back pain
- swelling of the legs or arms
- pain or tenderness to the touch in the legs
- redness or color change of legs or arms
- severe changes in vision
Signs of myocardial infarction (infrequent) include
- chest pain or tightness (which may extend to the arms, jaw, neck, and back)
- difficulty breathing
- cold sweat
- sudden fainting or dizziness
Other Adverse Effectsthat have been observed with XELJANZ are listed below.
Frequent(may affect up to 1 in 10 patients): lung infections (pneumonia and bronchitis), shingles, infections in the nasal passages, throat, or trachea (nasopharyngitis), flu, sinusitis, urinary bladder infection (cystitis), sore throat (pharyngitis), increased muscle enzymes in blood (signs of muscle problems), stomach pain (abdominal pain) (which may be due to stomach lining inflammation), vomiting, diarrhea, discomfort (nausea), indigestion, low white blood cell count, low red blood cell count (anemia), swelling of feet and hands, headache, high blood pressure (hypertension), cough, skin rash.
Infrequent(may affect up to 1 in 100 patients): lung cancer, tuberculosis, kidney infection, skin infection, simple herpes or oral ulcers (cold sores), increased creatinine in blood (a possible sign of kidney problems), increased cholesterol (including increased LDL), fever, fatigue (tiredness), weight gain, dehydration, muscle strain, tendonitis, joint swelling, joint sprain, abnormal sensations, insufficient sleep, sinus congestion, shortness of breath or difficulty breathing, skin redness, itching, fatty liver, painful inflammation of the small pouches protruding from the inner lining of the intestine (diverticulitis), viral infections, viral infections affecting the intestine, some types of non-melanoma skin cancer.
Rare(may affect up to 1 in 1,000 patients): blood infection (sepsis), lymphoma (white blood cell cancer), disseminated tuberculosis affecting bones and other organs, other unusual infections, joint infection, increased liver enzymes in blood (sign of liver problems), muscle and joint pain.
Very Rare(may affect up to 1 in 10,000 patients): tuberculosis affecting the brain and spinal cord, meningitis, infection of soft tissues and fascia.
In general, fewer adverse effects were observed in rheumatoid arthritis when XELJANZ was administered alone than in combination with methotrexate.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of XELJANZ
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging or on the bottle. The expiration date is the last day of the month indicated.
This medicine does not require any special storage temperature.
Store in the original bottle and packaging to protect it from light.
Discard after 60 days from the first opening
Do not use this medicine if you notice that the solution shows visible signs of deterioration.
Medicines should not be thrown away through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
XELJANZ Composition
- The active ingredient is tofacitinib.
- Each ml contains 1 mg of tofacitinib (as tofacitinib citrate).
- The other components are grape flavor [containing propylene glycol (E1520) (see section 2 "XELJANZ contains propylene glycol"), glycerin (E422), and natural flavors], hydrochloric acid, lactic acid (E270), purified water, sodium benzoate (E211) (see section 2 "XELJANZ contains sodium benzoate" and "XELJANZ contains sodium"), sucralose (E955), and xylitol (E967).
Product Appearance and Package Contents
XELJANZ 1 mg/ml oral solution is a clear, colorless solution.
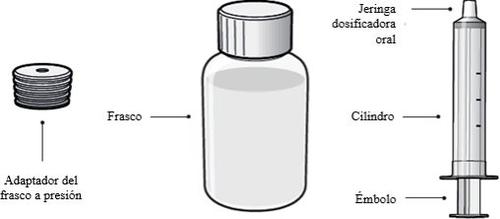
The 1 mg/ml oral solution is presented in 250 ml white HDPE bottles containing 240 ml of oral solution. Each package contains an HDPE bottle, a bottle adapter, and an oral dosing syringe with 3.2 ml, 4 ml, and 5 ml graduations.
Marketing Authorization Holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelles
Belgium
Manufacturer
Pfizer Service Company BVBA
Hoge Wei 10
1930 Zaventem
Belgium
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België /Belgique / Belgien Luxembourg/Luxemburg België /Belgique / Belgien Pfizer S.A./N.V. Tél/Tel: +32 (0)2 554 62 11 Luxembourg/Luxemburg Pfizer S.A. Tél/Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel. +3705 2514000 |
| Magyarország Pfizer Kft. Tel.: +36 1 488 37 00 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +35621 344610 |
Danmark Pfizer ApS Tlf: +45 44 20 11 00 | Nederland Pfizer bv Tel: +31 (0)10 406 43 01 |
Deutschland Pfizer Pharma GmbH Tel: +49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Ελλáδα PFIZER ΕΛΛΑΣ A.E. Τηλ.: +30 210 67 85 800 | Polska Pfizer Polska Sp. z o.o., Tel.: +48 22 335 61 00 |
España Pfizer, S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L. Tel: +40 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel.: +386 (0) 1 52 11 400 |
Ireland Pfizer Healthcare Ireland Tel: 1800 633 363 (toll free) +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: +421-2-3355 5500 |
Ísland Icepharma hf. Sími: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Κúπρος PFIZER ΕΛΛΑΣ Α.Ε. (CYPRUS BRANCH) Τηλ: +357 22 817690 | United Kingdom (Northern Ireland) Pfizer Limited Tel: +44 (0)1304 616161 |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel.: +371 670 35 775 |
Date of the Last Revision of this Prospectus:
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Instructions for the Use of XELJANZ Oral Solution
Read these Instructions for Use before starting to take XELJANZ oral solution. There may be new information.
Important Information about the Dosage of XELJANZ Oral Solution
Always use the oral dosing syringe provided with XELJANZ oral solution to measure and administer the prescribed dose. If you are not sure, ask your healthcare professional or pharmacist to show you how to measure the prescribed dose.
How should XELJANZ be stored?
Keep this medicine out of sight and reach of children.
Discard the remaining XELJANZ oral solution 60 days after the first opening.
To help you remember when you need to discard the XELJANZ bottle, you can write the date of the first use on the packaging and then:
Date of first use ____/____/____.
Before each use:
Wash your hands with water and soap and place the contents of the box on a clean and flat surface.
Each XELJANZ oral solution package contains
- 1 bottle adapter
- 1 XELJANZ oral solution bottle
- 1 oral dosing syringe
Step 1. Remove the bottle from the packaging
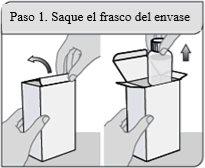
Remove the XELJANZ oral solution bottle from the packaging.
Step 2. Open the bottle
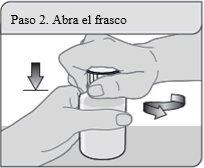
Open the bottle. Remove the sealed cap from the top of the bottle (only the first time).
Do not throw away the child-resistant cap.
Note:It is not necessary to shake the bottle before use.
Step 3. Insert the bottle adapter
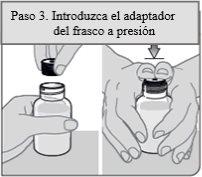
Remove the bottle adapter and the oral dosing syringe from the plastic wrapping. With the bottle on a flat surface, push the notched end of the bottle adapter into the neck of the bottle with your thumbs until it reaches the bottom of the bottle neck while holding the bottle firmly.
Note:Do not remove the bottle adapter after it has been inserted.
Step 4. Remove air from the dosing syringe
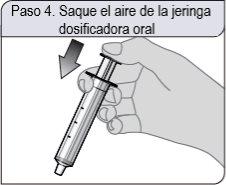
Push the plunger of the oral dosing syringe completely towards the end of the syringe cylinder to remove excess air.
Step 5. Insert the dosing syringe
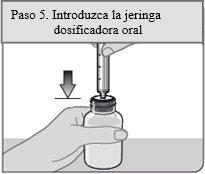
Insert the oral dosing syringe vertically into the bottle through the opening of the bottle adapter until it is firmly in place.
Step 6. Withdraw the dose from the bottle
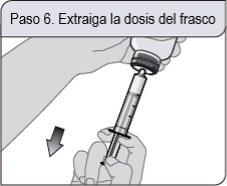
With the oral dosing syringe in place, turn the bottle upside down. Pull the plunger back.
If you see air bubbles in the oral dosing syringe, press the plunger completely to return the oral solution to the bottle. Then, withdraw the prescribed dose of oral solution.
Step 7. Remove the dosing syringe
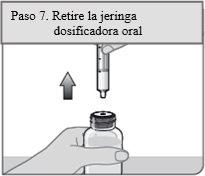
Place the bottle in an upright position and put it on a flat surface. Remove the oral dosing syringe from the bottle adapter by pulling the syringe cylinder upwards.
Step 8. Check the withdrawn dose
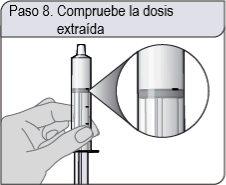
Check that you have withdrawn the correct dose in the oral dosing syringe.
If the dose is not correct, firmly insert the end of the oral dosing syringe into the bottle adapter. Push the plunger completely to return the oral solution to the bottle. Repeat steps 6 and 7.
Step 9. Take the XELJANZ dose
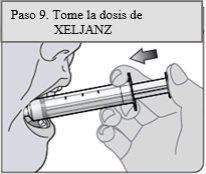
Place the tip of the oral dosing syringe inside the patient's cheek.
Slowly push the plunger to the bottom to administer all the medicine in the oral dosing syringe. Make sure the patient has time to swallow the medicine.
Step 10. Close the bottle
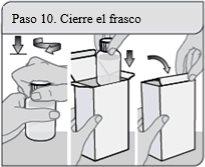
Close the bottle tightly by turning the child-resistant cap clockwise. Leave the bottle adapter in place.
Put the bottle back in the packaging and close it to protect the XELJANZ oral solution from light.
Step 11. Clean the dosing syringe
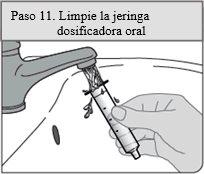
Remove the plunger from the syringe cylinder by pulling the plunger and cylinder in different directions.
Rinse both with water after each use.
Let them air dry; then put the oral dosing syringe back with the XELJANZ oral solution in the packaging.
Do not discard the oral dosing syringe.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to XELJANZ 1 mg/mL ORAL SOLUTIONDosage form: TABLET, 10 mgActive substance: tofacitinibManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: MODIFIED-RELEASE TABLET, 11 mgActive substance: tofacitinibManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: TABLET, 5 mgActive substance: tofacitinibManufacturer: Pfizer Europe Ma EeigPrescription required
Online doctors for XELJANZ 1 mg/mL ORAL SOLUTION
Discuss questions about XELJANZ 1 mg/mL ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







