XARELTO 1 mg/ml ORAL SUSPENSION GRANULES

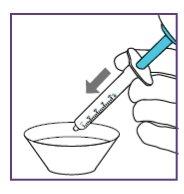
How to use XARELTO 1 mg/ml ORAL SUSPENSION GRANULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Xarelto 1mg/ml oral suspension
rivaroxaban
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. This leaflet is written for the patient (“you”) and for the parent or caregiver who will be giving this medicine to the child.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you or the child only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you or the child gets any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Xarelto is and what it is used for
- What you need to know before you take or give Xarelto
- How to take or give Xarelto
- Possible side effects
- Storing Xarelto
- Contents of the pack and other information
1. What Xarelto is and what it is used for
Xarelto contains the active substance rivaroxaban.
Xarelto belongs to a group of medicines called antithrombotic agents. It works by blocking a factor in the blood (factor Xa) and so reduces the tendency of the blood to form clots.
Xarelto is used in term newborns, infants, children and adolescents under 18 years of age to:
- treat blood clots and prevent them from happening again in the veins or blood vessels in the lungs, after at least 5 days of initial treatment with injectable medicines used to treat blood clots.
Read and follow the Instructions for Use provided with this medicine, as they will show you how to prepare and take or give Xarelto oral suspension.
2. What you need to know before you take or give Xarelto
Do not take or give Xareltoif you or the child
- are allergic to rivaroxaban or any of the other ingredients of this medicine (listed in section 6)
- have a condition that increases the risk of bleeding, such as bleeding disorders or active ulcerative gastrointestinal disease
- have a liver disease associated with an increased risk of bleeding
- are pregnant or breastfeeding.
Do not take or give Xarelto and inform your doctorif any of these apply to you or the child.
Warnings and precautions
Tell your doctor or pharmacist before you start taking Xarelto if:
- you or the child have an increased risk of bleeding. This may occur in the following situations:
- moderate or severe kidney problems, as kidney function may affect the amount of medicine that works in the body
- you or the child are taking other medicines to prevent blood clots (e.g. warfarin, dabigatran, apixaban or heparin), if these are absolutely necessary (see section “Do not take or give Xarelto”)
- bleeding disorders
- uncontrolled high blood pressure
- stomach or intestinal diseases that may cause bleeding, such as inflammatory bowel disease or stomach ulcers
- problems with blood vessels in the eyes (retinopathy)
- a lung disease where the airways are widened and filled with pus (bronchiectasis) or previous lung bleeding
- you or the child have a heart valve replacement
- you or the child have a disease called antiphospholipid syndrome (a disorder of the immune system that increases the risk of blood clots)
- you or the child have unstable blood pressure
- you or the child are scheduled to receive another treatment or undergo a procedure to remove a blood clot from the lungs
Tell your doctorif you or the child have any of these conditions before taking or giving Xarelto. The doctor will decide if you or the child should be treated with this medicine and be kept under closer observation.
Do not giveXarelto to children under 6 months of age who:
- were born before 37 weeks of gestation, or
- weigh less than 2.6 kg, or
- have been breastfed or formula-fed for less than 10 days
In these cases, the dose of Xarelto cannot be reliably determined and has not been studied in these children.
If you or the child need surgery
- It is very important to take or give Xarelto before and after surgery, exactly at the times indicated by your doctor.
- If the operation requires the insertion of a catheter or injection into the spine (e.g. for epidural or spinal anesthesia, or pain relief):
- it is very important to take or give Xarelto before and after the injection or removal of the catheter, exactly at the times indicated by your doctor.
- tell your doctor immediately if you or the child experience numbness or weakness in the legs or problems with the bowel or bladder after anesthesia. In this case, urgent attention is needed.
Children and adolescents
Xarelto oral suspension should be used in patients under 18 years of age to treat blood clots and prevent them from happening again in the veins or blood vessels in the lungs. There is not enough information on its use in children and adolescents for other conditions.
Other medicines and Xarelto
Tell your doctor or pharmacist if you or the child are taking, have recently taken or might take any other medicines, including those obtained without a prescription.
- If you or the child are taking:
- any medicine for a fungal infection (e.g. fluconazole, itraconazole, voriconazole, posaconazole), unless it is only applied to the skin
- ketoconazole tablets (used to treat Cushing's syndrome, where the body produces too much cortisol)
- any medicine for bacterial infections (e.g. clarithromycin, erythromycin)
- any medicine for HIV/AIDS (e.g. ritonavir)
- other medicines to reduce blood clotting (e.g. enoxaparin, clopidogrel or vitamin K antagonists, such as warfarin or acenocoumarol)
- medicines to relieve inflammation and pain (e.g. naproxen or acetylsalicylic acid)
- dronedarone, a medicine for irregular heartbeat
- certain medicines for depression (selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SNRIs))
If any of these apply to you or the child, tell your doctorbefore taking or giving Xarelto, as the effect of Xarelto may be increased. Your doctor will decide if you or the child should be treated with this medicine and be kept under closer observation.
If the doctor thinks that you or the child are at a higher risk of developing a stomach or intestinal ulcer, preventive treatment may be necessary.
- If you or the child are taking:
- any medicine for epilepsy(phenytoin, carbamazepine, phenobarbital)
- St. John's Wort (Hypericum perforatum),a herbal medicine for depression
- rifampicin,an antibiotic.
If any of these apply to you or the child, tell your doctorbefore taking or giving Xarelto, as the effect of Xarelto may be reduced. The doctor will decide if you or the child should be treated with this medicine and be kept under closer observation.
Pregnancy and breastfeeding
- If you or the adolescent are pregnant or breastfeeding, do not take or give Xarelto.
- If there is a possibilitythat you or the adolescent may become pregnant, a reliable contraceptiveshould be used while taking Xarelto.
- If you or the adolescent become pregnant while taking this medicine, tell your doctor immediately, who will decide how to continue treatment.
Driving and using machines
Xarelto may cause dizziness or fainting. You or the child should not drive, ride a bicycle or use tools or machines if you are affected by these symptoms.
Xarelto contains sodium benzoate and sodium
This medicine contains 1.8 mg of sodium benzoate (E 211) in each ml of oral suspension. Sodium benzoate (E 211) may increase the risk of jaundice (yellowing of the skin and eyes) in newborns (up to 4 weeks of age).
This medicine contains less than 1 mmol of sodium (23 mg) per milliliter; this is, essentially “sodium-free”.
3. How to take or give Xarelto
Follow exactly the administration instructions for this medicine given by your doctor for you or the child. If you are unsure, consult your doctor or pharmacist again.
Make sure the correct information about how much and how often to take or give Xarelto is written in the designated area of the box. If it is not, ask your pharmacist or doctor to provide the relevant information.
Instructions for use
To know how to prepare and take or give the Xarelto oral suspension:
- Read the Instructions for Use leaflet that comes with the pack and
- Watch the educational video that you can access through the QR code on the patient information card provided with this medicine.
How to take or give
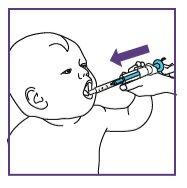
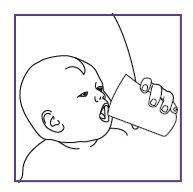
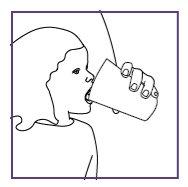
Take or give Xarelto oral suspension with food (breast milk or formula) or with a meal. Each dose of Xarelto should be swallowed with a normal amount of liquid (e.g. 20 ml in children from 6 months to 240 ml in adolescents). This normal amount can include a usual amount of drink used for feeding (e.g. breast milk, formula, nutritional drink).
Your doctor may also give the oral suspension through a tube inserted into the stomach.
How much to take or give
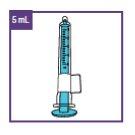
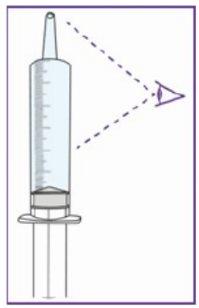
The dose of Xarelto depends on the patient's body weight. The doctor will calculate it as a volume (in milliliters, ml) of the oral suspension. This should be measured using the blue syringe (either 1 ml, 5 ml or 10 ml, see Table 1) provided with this medicine. Your doctor will prescribe the required volume, including the specific syringe to use.
Your doctor will tell you how much of the oral suspension you or the child should take.
The following table is the one your doctor will use. Do not adjust the dose yourself.
All materials to prepare and administer the oral suspension are provided with the medicine (except for tap water). Use only still water to avoid bubbles. Use only the provided syringeto administer Xarelto to ensure exact dosing. Do not use any other method to administer the solution, such as an alternative syringe, a spoon, etc.
Since the dose of Xarelto is based on body weight, it is important to attend scheduled visits with the doctor, as the dose may need to be adjusted as the weight changes, especially in children under 12 kg. This ensures that the child receives the correct dose of Xarelto.
Table 1: Recommended dose of Xarelto in children
Body weight [kg] | Individual dose* | Daily frequency of administration | Total daily dose* | Suitable blue syringe |
2.6 to less than 3 | 0.8 ml | 3 times | 2.4 ml | 1 ml |
3 to less than 4 | 0.9 ml | 2.7 ml | ||
4 to less than 5 | 1.4 ml | 4.2 ml | 5 ml | |
5 to less than 7 | 1.6 ml | 4.8 ml | ||
7 to less than 8 | 1.8 ml | 5.4 ml | ||
8 to less than 9 | 2.4 ml | 7.2 ml | ||
9 to less than 10 | 2.8 ml | 8.4 ml | ||
10 to less than 12 | 3.0 ml | 9.0 ml | ||
12 to less than 30 | 5.0 ml | 2 times | 10.0 ml | 5 ml or 10 ml |
30 to less than 50 | 15.0 ml | once | 15.0 ml | 10 ml |
50 or more | 20.0 ml | 20.0 ml | ||
|
Your doctor may also prescribe tablets if you or the child are able to swallow the tablet and weigh at least 30 kg.
When to take or give Xarelto
Take or give the oral suspension as indicated by your doctor every day until the doctor tells you to stop.
Take or give the oral suspension at the same time every day to help you remember. Consider setting an alarm to remind you.
Please watch the child to make sure they take the whole dose.
If the doctor has told you to take or give the dose of Xarelto:
- once a day, do so with approximately 24 hours between doses
- twice a day, do so with approximately 12 hours between doses
- three times a day, do so with approximately 8 hours between doses
Your doctor will decide how long you or the child should continue treatment.
If you or the child spit out the dose or vomit
- less than 30 minutes after taking Xarelto, take or give a new dose.
- more than 30 minutes after taking Xarelto, do nottake or give a new dose. Continue taking or giving the next dose of Xarelto at the next scheduled time.
Call your doctor if you or the child repeatedly spit out the dose or vomit after taking Xarelto.
If you forget to take or give Xarelto
- If you are taking or giving Xarelto once a day, take or give the missed dose as soon as you remember on the same day. If this is not possible, skip that dose. Then take or give the next dose of Xarelto the next day. Do not take or give more than one dose per day.
- If you are taking or giving Xarelto twice a day:
- Missed morning dose: take or give the missed dose as soon as you remember. You can take or give it with the evening dose.
- Missed evening dose: you can take or give the missed dose only on the same evening. Do not take or give two doses the next morning.
- If you are taking or giving Xarelto three times a day, do not replace the missed dose. Continue with the next scheduled dose (every 8 hours).
The next day, after a missed dose, continue as prescribed by the doctor, one, two or three times a day.
If you take or give too much Xarelto
Call your doctor immediately if you have taken or given too much Xarelto oral suspension. Taking or giving too much Xarelto increases the risk of bleeding.
If you stop treatment with Xarelto
Do not stop treatment with Xarelto without consulting your doctor first, as Xarelto treats and prevents serious conditions.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Like other similar medicines to reduce blood clot formation, Xarelto can cause bleeding that can endanger the patient's life. Excessive bleeding can cause a sudden drop in blood pressure (shock). In some cases, the bleeding may not be evident.
Tell your doctor immediatelyif you or the child experience any of the following adverse effects:
- Signs of bleeding
- bleeding in the brain or inside the skull (symptoms may include headache, weakness on one side of the body, vomiting, seizures, decreased level of consciousness, and stiffness in the neck. This is a serious medical emergency. Go to the doctor immediately!)
- prolonged or excessive bleeding
- exceptional weakness, fatigue, paleness, dizziness, headache, unexplained swelling, difficulty breathing, chest pain or angina
Your doctor may decide to keep you or the child under closer observation or change the treatment.
- Signs of severe skin reactions
- intense skin rashes that spread, blisters, or lesions on the mucous membranes, e.g., in the mouth or eyes (Stevens-Johnson syndrome/toxic epidermal necrolysis)
- drug reaction that causes rash, fever, inflammation of internal organs, blood abnormalities, and systemic disease (DRESS syndrome).
The frequency of these adverse effects is very rare (up to 1 in 10,000 people).
- Signs of severe allergic reactions
- swelling of the face, lips, mouth, tongue, or throat; difficulty swallowing; hives, and difficulty breathing; sudden drop in blood pressure.
The frequencies of severe allergic reactions are very rare (anaphylactic reactions, including anaphylactic shock; may affect up to 1 in 10,000 people) and uncommon (angioedema and allergic edema; may affect up to 1 in 100 people).
General list of possible adverse effects found in adults and children and adolescents:
Frequent(may affect up to 1 in 10 people)
- decrease in red blood cells that can cause paleness and weakness or difficulty breathing
- bleeding in the stomach or intestine, urogenital hemorrhage (including blood in the urine and heavy menstrual bleeding), nasal bleeding, bleeding gums
- bleeding in the eye (including bleeding in the white part of the eye)
- bleeding into a tissue or cavity of the body (hematoma, bruising)
- coughing up blood
- bleeding from the skin or under the skin
- bleeding after surgery
- oozing of blood or fluid from a surgical wound
- swelling of the limbs
- pain in the limbs
- alteration of kidney function (may be seen in tests performed by the doctor)
- fever
- stomach pain, indigestion, dizziness or feeling of dizziness, constipation, diarrhea
- low blood pressure (symptoms may be dizziness or fainting when standing up)
- general decrease in strength and energy (weakness, fatigue), headache, dizziness,
- rash, itching of the skin
- blood tests may show an increase in some liver enzymes
Uncommon(may affect up to 1 in 100 people)
- bleeding in the brain or inside the skull (see above, possible adverse effects that may be a sign of bleeding)
- bleeding in a joint, which causes pain and swelling.
- thrombocytopenia (low platelet count, cells that help blood clotting)
- allergic reaction, including skin allergic reaction
- alteration of liver function (may be seen in tests performed by the doctor)
- blood tests may show an increase in bilirubin, some pancreatic or liver enzymes, or platelet count
- fainting
- feeling of discomfort
- increased heart rate
- dry mouth
- hives
Rare(may affect up to 1 in 1,000 people)
- bleeding in a muscle
- cholestasis (decreased bile flow), hepatitis, including traumatic hepatocellular injury (inflammation or liver damage) yellowing of the skin and eyes (jaundice)
- localized swelling
- blood accumulation (hematoma) in the groin after a complication in heart surgery where a catheter is inserted into the leg artery (pseudoaneurysm)
Very Rare(may affect up to 1 in 10,000 people)
- accumulation of eosinophils, a type of granulocytic white blood cells that cause inflammation in the lung (eosinophilic pneumonia).
Frequency not known(frequency cannot be estimated from available data)
- renal failure after severe bleeding
- bleeding in the kidney, sometimes with blood in the urine, which causes the kidneys to malfunction (anticoagulant-related nephropathy)
- increased pressure in the muscles of the legs or arms after bleeding, which causes pain, swelling, altered sensation, numbness, or paralysis (compartment syndrome after bleeding)
Adverse effects in children and adolescents
In general, the adverse effects observed in children and adolescents treated with Xarelto were similar to those observed in adults and their severity was mainly mild to moderate.
Adverse effects that were more frequently observed in children and adolescents:
Very frequent(may affect more than 1 in 10 people)
- headache
- fever
- nasal bleeding
- vomiting
Frequent(may affect up to 1 in 10 people)
- accelerated heartbeats
- blood tests may show an increase in bilirubin (bile pigment)
- thrombocytopenia (low platelet count, cells that help blood clotting)
- heavy menstrual bleeding
Uncommon(may affect up to 1 in 100 people)
- blood tests may show an increase in a subcategory of bilirubin (direct bilirubin, bile pigment)
Reporting adverse effects
If you or the child experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Xarelto
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging and on the bottle after "EXP" or "CAD".
The expiration date is the last day of the month indicated.
After preparation, the validity period of the suspension is 14 days at room temperature.
Do not store above 30°C.
Do not freeze. Store the prepared suspension in an upright position.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
Xarelto Composition
- The active ingredient is rivaroxaban. A glass vial contains
- 51.7 mg of rivaroxaban, for the addition of 50 ml of water,
- or 103.4 mg of rivaroxaban, for the addition of 100 ml of water.
After preparation, each ml of the oral suspension contains 1 mg of rivaroxaban.
- The other components are:
- Citric acid, anhydrous (E 330), hypromellose (2910), mannitol (E 421), microcrystalline cellulose, sodium carboxymethylcellulose, sodium benzoate (E 211) (see section 2 "Xarelto contains sodium benzoate and sodium"), sucralose (E 955), xanthan gum (E 415), sweet and creamy flavor (constituted by flavoring substances, maltodextrin [corn], propylene glycol [E 1520] and acacia gum [E 414]).
Product Appearance and Container Contents
Xarelto granules for oral suspension are a white granule in a glass vial with a child-resistant screw cap.
Package sizes
- For children who weigh less than 4kg:
Foldable box with a brown glass vial containing 2.625 g of granules (equivalent to 51.7 mg of rivaroxaban), two 1 ml blue syringes, a 50 ml water syringe, and an adapter.
- For children who weigh 4kg or more:
Foldable box with a brown glass vial containing 5.25 g of granules (equivalent to 103.4 mg of rivaroxaban), two 5 ml blue syringes and two 10 ml blue syringes, a 100 ml water syringe, and an adapter.
Only some package sizes may be marketed.
The volume and frequency of the adjusted dose based on individual weight must be specified by the prescribing doctor. They must be written on the outer box when provided to parents, caregivers, or patients.
Follow the Instructions for Use leaflet carefully, which is supplied with each package.
Watch the educational video that can be accessed through the QR code on the patient information card provided with the medication.
Marketing Authorization Holder and Manufacturer
Bayer AG
51368 Leverkusen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium / Belgium / Belgium Bayer SA-NV Tel: +32-(0)2-535 63 11 | Lithuania UAB Bayer Tel: +370-5-233 68 68 |
| Luxembourg / Luxembourg Bayer SA-NV Tel: +32-(0)2-535 63 11 |
Czech Republic Bayer s.r.o. Tel: +420-266 101 111 | Hungary Bayer Hungária KFT Tel: +36-1-487 4100 |
Denmark Bayer A/S Tel: +45-45 235 000 | Malta Alfred Gera and Sons Ltd. Tel: +356-21 44 62 05 |
Germany Bayer Vital GmbH Tel: +49-(0)214-30 513 48 | Netherlands Bayer B.V. Tel: +31–(0)297-28 06 66 |
Estonia Bayer OÜ Tel: +372-655 85 65 | Norway Bayer AS Tel: +47-23 13 05 00 |
Greece Bayer Ελλάς ΑΒΕΕ Tel: +30-210-618 75 00 | Austria Bayer Austria Ges. m. b. H. Tel: +43-(0)1-711 460 |
Spain Bayer Hispania S.L. Tel: +34-93-495 65 00 | Poland Bayer Sp. z o.o. Tel: +48-22-572 35 00 |
France Bayer HealthCare Tel (Green Number): +33-(0) 800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351-21-416 42 00 |
Croatia Bayer d.o.o. Tel: + 385-(0)1-6599 900 | Romania SC Bayer SRL Tel: +40-(0)21-528 59 00 |
Ireland Bayer Limited Tel: +353 1 216 3300 | Slovenia Bayer d. o. o. Tel: +386-(0)1-58 14 400 |
Iceland Icepharma hf. Tel: +354-540 80 00 | Slovak Republic Bayer, spol. s r.o. Tel: +421-(0)2-59 21 31 11 |
Italy Bayer S.p.A. Tel: +39-02-3978 1 | Finland Bayer Oy Tel: +358-(0)20-78521 |
Cyprus NOVAGEM Limited Tel: +357-22-48 38 58 | Sweden Bayer AB Tel: +46-(0)8-580 223 00 |
Latvia SIA Bayer Tel: +371-67 84 55 63 | United Kingdom Bayer plc Tel: +44-(0)118 206 3000 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu.
Instructions for Use (IFU)
Instructions for Use
Xarelto 1mg/ml
100 ml vial with 2.625 g of granules for the preparation of an oral suspension
Active Pharmaceutical Ingredient: rivaroxaban
Preparation and Administration of the Oral Suspension (mixture of granules and water)
Glossary and Symbols
- Granules: powder (supplied in the vial) that contains the active pharmaceutical ingredient.
- Water syringe: 50 ml syringe used to measure and add 50 ml of water to the vial containing the Xarelto granules.
- Suspension: mixture of granules and water (for oral use).
- Blue syringe: syringe with a blue plunger for extracting and administering Xarelto orally.


Before Starting
- Read all sections of these Instructions for Use carefully before using Xarelto for the first time and before administering each dose.
- Watch the educational video that can be accessed through the QR code included in the patient information card provided with the medication.
- Make sure you understand the instructions before starting. If not, call your doctor.
- For more information about Xarelto, consult the leaflet.
Package Contents
Each Xarelto box contains the following components:
| 1vial with a child-resistant screw capcontaining the Xarelto granules |
| 150 ml water syringe(for single use) |
| 1vial adapter |
| 21 ml blue syringes |
| 1copy of the Instructions for Use (IFU)(this document) |
| 1leaflet Provides important information about Xarelto. |
| 1patient information card Important information in case of emergency. The patient should carry it at all times and present it to each doctor or dentist before treatment. |

Warnings and Precautions
- Use onlypotable water without gas to prepare the suspension to avoid the formation of bubbles. This means you can use
- tap water or
- non-carbonated mineral water (without gas)
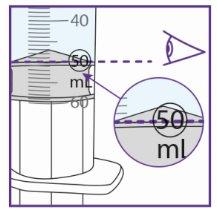
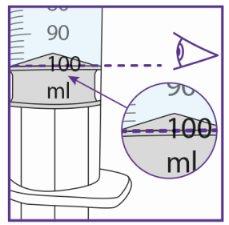
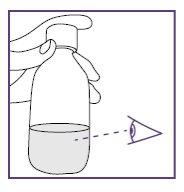
- It is very important to add the exact amount of water to the vial granules to ensure the correct concentration of Xarelto.
- Use the water syringe to measure 50 ml of water, see below for more information.
- Measure the amount of water you are going to provide to the vial very carefully.
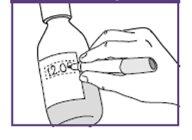
- After preparation, the suspension can be used for 14 days if stored at room temperature. Make sure to write the expiration date of the suspension (preparation date plus 14 days) in the field for this purpose on the vial label.
- Do notstore the suspension at a temperature above 30°C. Do notfreeze. If the suspension has been stored in the refrigerator, let it reach room temperature before extracting the corresponding dose.
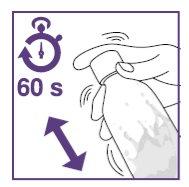
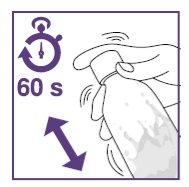
- Shake the suspension for the initial preparation for at least 60seconds.
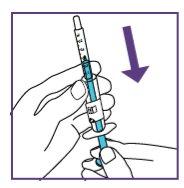
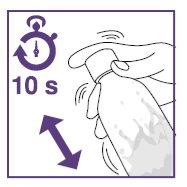
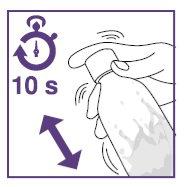
- Shake the suspension in the vial for at least 10secondsbefore each administration.
- It is very important that the prescribed volume of the Xarelto dose is administered.
- Make sure you know the prescribed dose and frequency of administration. Ask your doctor or pharmacist if you do not know the prescribed dose and its frequency.
- Carefully adjust the blue syringe according to the prescribed volume.
- Administer the prescribed dose using the blue syringe. Follow your doctor's instructions on the daily frequency with which you should administer the prescribed dose.
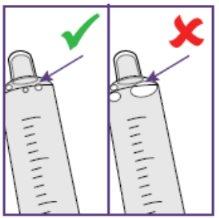
- Check that there are no air bubbles in the blue syringe before administering the oral suspension.
- If your child does not repeatedly take the entire required dose or spits out part of it, call your child's doctor to find out what to do.
- Between doses, keep the oral suspension out of sight and reach of children.
- Keep the Instructions for Use to be able to consult them later during the use of Xarelto.
Using Xarelto
- The Xarelto suspension is for oral use only.
- The volume and frequency of Xarelto administration depend on your child's weight, so they will change over time if your child receives Xarelto for an extended period.
- Your child's doctor will indicate the correct dose volume.
- Do notchange the dose on your own.
- Alwaysuse the volume prescribed by your child's doctor and have the administration dose written on the designated field on the outside of the box.
If it is not written in this field, ask your child's doctor or pharmacist to provide the relevant information.
- Follow the detailed Instructions for Use that appear in the following sections.
Be careful to follow the administration instructions (see leaflet):
- Preparation of the oral suspension
Step1.1: Preparation – All set
The preparation of the suspension is done once with each new package.
Before preparing the suspension:
|
|
|
Do notuse the medication if it has already expired. |
| |
|
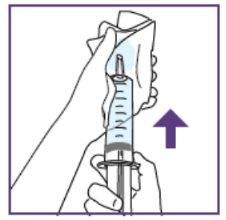
Step1.2: Filling the required water volume
Each time you start a new package, use only the new materials included in the new package.
|
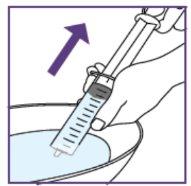
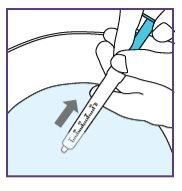
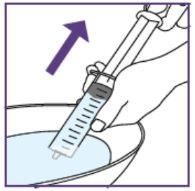
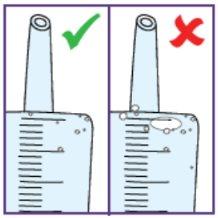
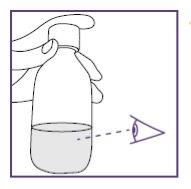
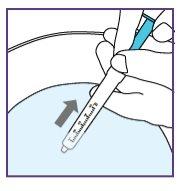
To do this, pull the plunger rod towards you and make sure the opening of the water syringe remains below the surface of the water at all times. This will avoid air bubbles in the syringe.
Tap with your fingers to move the air bubbles even further to the top.
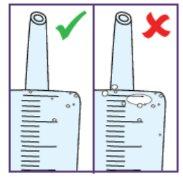
Small air bubbles are not critical, but large ones are critical. For more information on what to do, see below.
|
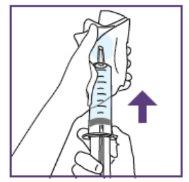
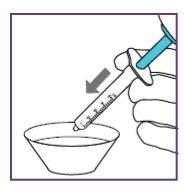
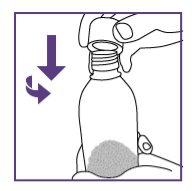
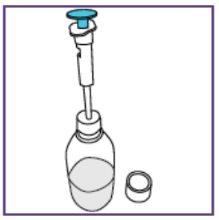
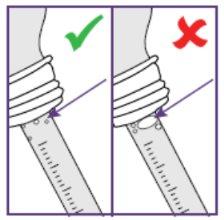
Step1.3: Adding water to the granules
|
|
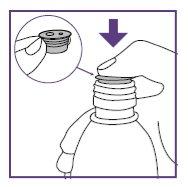
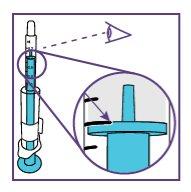
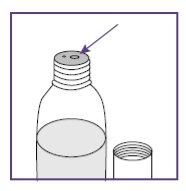
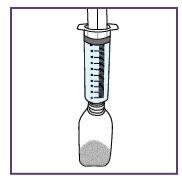
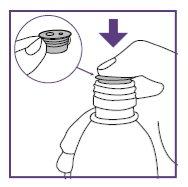
Step1.4: Placing the adapter and mixing the oral suspension
The adapter is used to fill the blue syringe with the suspension.
| |
|
|
|
|
|
|
- This is intended to obtain a well-mixed suspension.
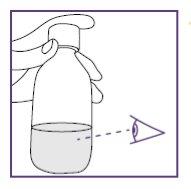
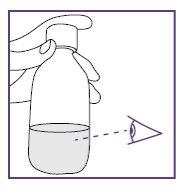
- Check that the suspension is well mixed:
- without lumps,
- without deposits.

- If there are lumps or sediment, repeat steps d to f.
- The suspension is ready for use when there are no lumps or deposits.
Do not add more water to the bottle.
The suspension has a validity period of 14 days at room temperature.
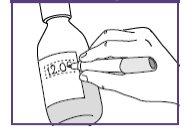
- Write the expiration date of the newly prepared suspension on the bottle label.
Preparation date+14days
The pictogram shown is only an example.
- Dose adjustment with each new blue syringe
To avoid overdosing or underdosing, an exact dose of suspension is required.
Before taking the first dose from the bottle, the attached blue syringe must be prepared according to the dose prescribed by your child's doctor. This information can be found in the area of the box dedicated to this effect. If no information has been entered here, consult with the child's doctor or pharmacist.
After setting the dose, the same blue syringe can be used for all administrations to be performed from the prepared suspension bottle in step 1.
Once the dose has been set on the blue syringe, it can no longer be changed.
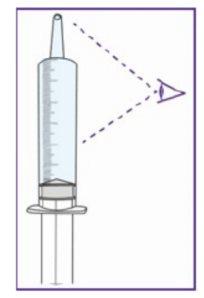
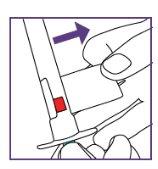
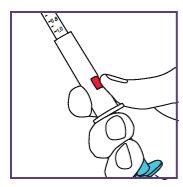
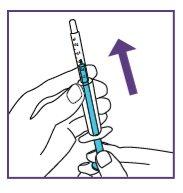
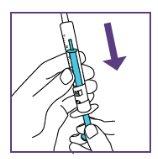
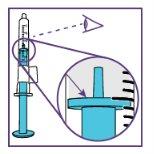
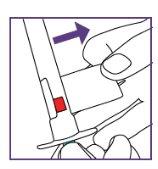
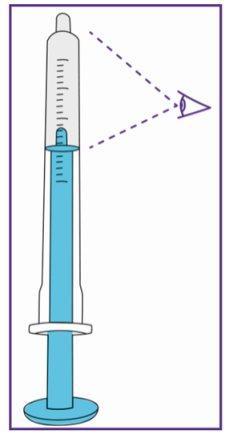
The blue syringe has a scale (ml). The scale of the 1 ml blue syringe starts with 0.2 ml. The graduation marks are in increments of 0.1 ml. Note: Do not remove the peel-off label until instructed in the Instructions for use. The blue syringe has a redbutton to adjust the volume. This button is initially covered by a peel-off label. The syringe volume is fixed by pressing the fixed red button, which can only be done once. Do notpress the redbutton until instructed in the Instructions for use. Once the redbutton has been pressed, the volume can no longer be adjusted. | |
|
|
Ask your pharmacist or doctor to provide it. | |
| |
|
When moving the plunger rod, you will hear a "click" for each adjustable volume step.
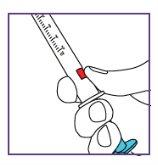
The pictogram shown is only an example. Your volume may be different. Be careful, do notpull the plunger beyond the volume to be administered. Be careful, do notpress the label when pulling the plunger. |
| |
|
Adjust it accordingly. |
|
The click sound will not be audible afterwards.
|
|
The blue syringe can now be used. |
- Administration of the oral suspension
Follow the steps described below for each administration needed.
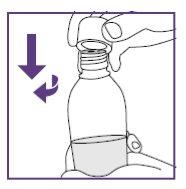
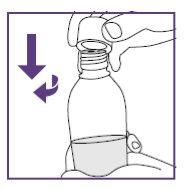
Step3.1: Mixing of the oral suspension

|
|
|
Repeat steps a and b. |
Let the bottle stand until the foam dissolves. | |
|
Note: The larger opening visible in the adapter is used to connect the blue syringe. The surface of the bottle adapter must be free of liquid.
Remove the liquid with a clean paper towel. |
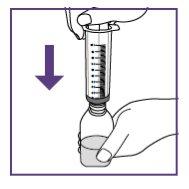
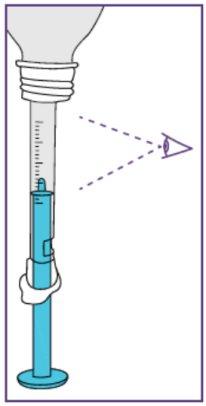
Step3.2: Extraction of the required dose




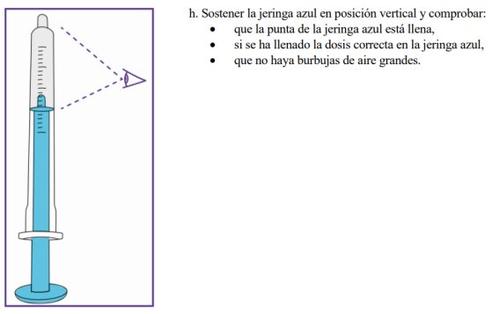

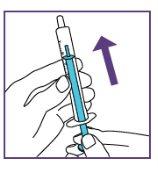
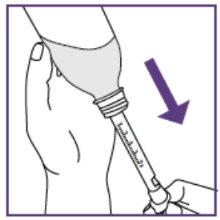
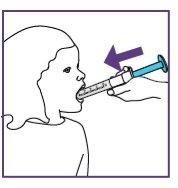
Step3.3: Administration of the prescribed dose
|
|
|
|
- Cleaning and storage
The blue syringe must be cleaned after each application.
Follow the steps indicated below to clean the device. A total of threecleaning cycles are required to ensure adequate cleaning.
Before starting, you will need the following materials for step 4.1:
|
Step4.1: Cleaning
|
|
|
|
| |
|
Step4.2: Conservation
Keep the blue syringe in a clean and dry place until its next use, e.g. store it in the Xarelto box provided.
Keep it away from sunlight.

Keep the suspension below 30 °C.

- Disposal
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Damage/Malfunction
Any serious incident that occurs in relation to the product must be reported to the manufacturer and the relevant authority in your country.
Instructions for use (IFU)
Instructions for use
Xarelto 1mg/ml
Bottle of 250ml with 5.25g of granulate for the preparation of an oral suspension
Active pharmaceutical ingredient: rivaroxaban
Preparation and administration of the oral suspension (mixing of granulate and water)
Glossary and symbols
- Granulate: powder (supplied in the bottle) that contains the active pharmaceutical ingredient.
- Water syringe: 100 ml syringe used to measure and add 100 ml of water to the bottle containing the Xarelto granulate.
- Suspension: mixture of granulate and water (for oral use).
- Blue syringe: syringe with blue plunger for extracting and orally administering Xarelto.


Before starting
- Read all sections of these Instructions for use carefully before using Xarelto for the first time and before administering each dose.
- Watch the educational video that can be accessed through the QRD code included in the patient information card provided with the medication.
- Make sure you understand the instructions before starting. If not, call your doctor.
- For more information about Xarelto, consult the package leaflet.
Package contents
Each Xarelto box contains the following components:
| 1bottle with a child-resistant screw capcontaining the Xarelto granulate |
| 1water syringe(for single use) |
| 1bottle adapter |
| 2blue syringes of 5ml 2blue syringes of 10ml |
| 1copy of the Instructions for use (IFU)(this document) |
| 1package leaflet Provides important information about Xarelto. |
| 1patient information card Important information in case of emergency. The patient must carry it at all times and present it to each doctor or dentist before treatment. |

Warnings and precautions
- Use onlygas-free potable water to prepare the suspension to avoid the formation of bubbles. This means you can use
- tap water or
- non-carbonated mineral water (without gas)
- It is very important to add the exact amount of water to the bottle granulate to ensure the correct concentration of Xarelto.
- Use the water syringe to measure 100 ml of water, see below for more information.
- Measure the amount of water you are going to provide to the bottle very carefully.
- After preparation, the suspension can be used for 14 days if stored at room temperature. Make sure to write the expiration date of the suspension (preparation date plus 14 days) in the field for this purpose on the bottle label.
- Do notstore the suspension at a temperature above 30 °C. Do notfreeze. If the suspension has been stored in the refrigerator, let it reach room temperature before extracting the corresponding dose.
- Shake the suspension for initial preparation for at least 60seconds.
- Shake the suspension in the bottle for at least 10secondsbefore each administration.
- It is very important that the prescribed volume of Xarelto is administered.
- Make sure you know the prescribed dose and frequency of administration. Ask your doctor or pharmacist if you do not know the prescribed dose and its frequency.
- Carefully adjust the blue syringe according to the prescribed volume.
- Administer the prescribed dose using the blue syringe. Follow your doctor's instructions on the daily frequency with which you should administer the prescribed dose.
- Check that there are no air bubbles in the blue syringe before administering the oral suspension.
- If your child does not repeatedly take the entire required dose or spits out part of it, call your child's doctor to find out what to do.
- Between doses, keep the oral suspension out of sight and reach of children.
- Keep the Instructions for use to be able to consult them later during the use of Xarelto.
Use of Xarelto
- Xarelto suspension is for oral use only.
- The volume and frequency of Xarelto administration depend on your child's weight, so they will change over time if your child receives Xarelto for an extended period.
- The child's doctor will indicate the correct dose volume and administration frequency.
- Do not change the dose on your own.
- Always use the volume prescribed by the child's doctor and have the correct dose and administration frequency written on the designated field on the outside of the box.
If it is not written in this field, ask the child's doctor or pharmacist to provide the relevant information.
- Follow the detailed Instructions for use outlined in the following sections.
- Be careful to follow the instructions related to administration:
- Preparation of the oral suspension
Step1.1: Preparation – All set
Preparation of the suspension is done once with each new package.
Before preparing the suspension:
|
|
|
Do notuse the medication if it has already expired. |
| |
|
Step1.2: Filling the required water volume
Each time you start a new package, use only the new materials included in the new package.
|
To do this, pull the plunger rod towards you and ensure the water syringe opening remains below the water surface at all times. This will avoid air bubbles in the syringe.
Small air bubbles are not critical, but large ones are critical. For more information on what to do, see below.
|
Step1.3: Adding water to the granules
| |
|
|
|
|
|
The entire volume of water must be transferred to the bottle.
|
Step1.4: Placing the adapter and mixing the oral suspension
The adapter is used to fill the blue syringe with the suspension.
| |
|
|
|
|
|
|
|
|
Do not add more water to the bottle. The suspension has a validity period of 14 days at room temperature. | |
|
Preparation date+14days The pictogram shown is just an example. |
- Adjusting the prescribed dose with each new blue syringe
To avoid overdosing or underdosing, an exact dose of suspension is required.
Before taking the first dose from the bottle, the attached blue syringe must be prepared according to the dose prescribed by the child's doctor. This information can be found in the area of the box dedicated to this purpose. If no information has been entered here, consult the child's doctor or pharmacist.
After setting the dose, the same blue syringe can be used for all administrations to be performed from the prepared suspension bottle in step 1.
Once the dose has been set on the blue syringe, it can no longer be changed.
Step2.1: Selecting a suitable blue syringe
This package includes dosing devices of different capacities:
Blue syringes of 5mlfor doses of 1mlto 5ml | |
Blue syringes of 10mlfor doses of 5mlto 10ml | |
The other blue syringes are not necessary.
Note: Do not remove the peel-off label until instructed in the Instructions for use. The blue syringe has a redbutton to adjust the volume. This button is initially covered by a peel-off label. The syringe volume is set by pressing the red button, which can only be done once. Do notpress the redbutton until instructed in the Instructions for use. Once the redbutton has been pressed, the volume can no longer be adjusted. | |
Step2.2: Adjusting the necessary dose on the new blue syringe The blue syringe has a scale (ml). The scale on the 5 ml blue syringe starts at 1 ml. The graduation marks are in increments of 0.2 ml. The scale on the 10 ml blue syringe starts at 2 ml. The graduation marks are in increments of 0.5 ml. | |
|
Note: Use the 10 ml blue syringe for prescribed doses over 10 ml as follows: Dose of 15 ml: 2 x 7.5 ml of the blue syringe Dose of 20 ml: 2 x 10 ml of the blue syringe |
Ask your pharmacist or doctor to provide it. | |
| |
|
The pictogram shown is just an example. Your volume may be different. Be careful, do notpull the plunger beyond the volume to be administered. Be careful, do notpress the label when pulling the plunger. |
| |
|
Adjust it accordingly. |
|
The click sound will not be audible afterwards.
|
|
The blue syringe is now ready for use. |
- Administration of the oral suspension
Follow the steps described below for each administration needed.
Step3.1: Mixing the oral suspension

|
|
|
Repeat steps a. and b. |
Let the bottle stand until the foam dissolves. | |
|
Note: The larger opening visible in the adapter is used to connect the blue syringe. The adapter surface on the bottle must be free of liquid.
Remove the liquid with a clean paper towel. |
Step3.2: Withdrawing the necessary dose
|
|
Step3.3: Administration of the prescribed dose
|
|
|
|
of liquid.
|
- Cleaning and storage
The blue syringe must be cleaned after each application.
Follow the steps indicated below to clean the device. In total, threecleaning cycles are needed to ensure adequate cleaning.
Before starting, you will need the following materials for step 4.1:
|
Step4.1: Cleaning
|
|
|
|
| |
|
Step4.2: Storage
Store the blue syringe in a clean and dry place until its next use, e.g., keep it in the Xarelto box provided.
Keep it away from sunlight.

Store the suspension below 30°C.

- Disposal
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to XARELTO 1 mg/ml ORAL SUSPENSION GRANULESDosage form: TABLET, 15 mgActive substance: rivaroxabanManufacturer: Egis Pharmaceuticals Plc.Prescription requiredDosage form: TABLET, 20 mgActive substance: rivaroxabanManufacturer: Egis Pharmaceuticals Plc.Prescription requiredDosage form: TABLET, 10 mgActive substance: rivaroxabanManufacturer: Towa Pharmaceutical Europe S.L.Prescription required
Online doctors for XARELTO 1 mg/ml ORAL SUSPENSION GRANULES
Discuss questions about XARELTO 1 mg/ml ORAL SUSPENSION GRANULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions