XALKORI 50 mg CAPSULES, GRANULES FOR OPENING

How to use XALKORI 50 mg CAPSULES, GRANULES FOR OPENING
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
XALKORI 20mg granules in capsules for opening
XALKORI 50mg granules in capsules for opening
XALKORI 150mg granules in capsules for opening
crizotinib
The words "you" and "your" are used to refer to both the patient and the caregiver of the pediatric patient.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is XALKORI and what is it used for
- What you need to know before you take XALKORI
- How to take XALKORI granules in capsules for opening
- Possible side effects
- Storage of XALKORI
- Contents of the pack and other information
- Instructions for use
1. What is XALKORI and what is it used for
XALKORI is a cancer medicine that contains crizotinib as the active substance, used to treat adults with a type of lung cancer called non-small cell lung cancer, which has a specific alteration or defect in a gene called anaplastic lymphoma kinase (ALK) or in a gene called ROS1.
XALKORI is used to treat children and adolescents (from ≥ 1 to <18 years of age) with a type tumor called anaplastic large cell lymphoma (alcl) or inflammatory myofibroblastic (imt) that has specific rearrangement defect in gene kinase (alk).< p>
XALKORI may be prescribed to children and adolescents to treat ALCL if previous treatment has not helped to stop the disease.
XALKORI may be prescribed to children and adolescents to treat IMT if surgical treatment has not helped to stop the disease.
You should only receive this medicine under the supervision of a doctor with experience in the treatment of cancer. If you have any questions about how XALKORI works or why it has been prescribed for you, ask your doctor.
2. What you need to know before you take XALKORI
Do not take XALKORI
- If you are allergic to crizotinib or any of the other ingredients of this medicine (listed in section 6, "Composition of XALKORI").
Warnings and precautions
Talk to your doctor before you start taking XALKORI:
- If you have moderate or severe liver disease.
- If you have ever had any lung problems. Some lung problems can get worse during treatment with XALKORI, as XALKORI can cause inflammation of the lungs during treatment. Tell your doctor right away if you have a new symptom or if any of your symptoms get worse, including difficulty breathing, shortness of breath, or cough with or without mucus, or fever.
- If you have had an electrocardiogram (ECG) that has shown a heart problem called QT interval prolongation.
- If you have a low heart rate.
- If you have ever had stomach or intestinal problems, such as holes (perforation), or have had diseases that cause inflammation in the abdomen (diverticulitis) or if the cancer has spread to the abdomen (metastasis).
- If you have vision problems (see flashes of light, blurred vision, or double vision).
- If you have severe kidney disease.
- If you are currently being treated with any other medicine, including those listed in the section "Other medicines and XALKORI".
If any of the above applies to you, tell your doctor.
Tell your doctor right away after taking XALKORI:
- If you experience severe stomach or abdominal pain, fever, chills, shortness of breath, rapid heartbeat, partial or complete loss of vision (in one or both eyes), or changes in bowel habits.
Children and adolescents
The indication for non-small cell lung cancer does not include children and adolescents. Do not give this medicine to children under 1 year of age with ALK-positive ALCL or ALK-positive IMT. XALKORI should be given to children and adolescents under the supervision of an adult.
Other medicines and XALKORI
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including herbal medicines and medicines bought without a prescription.
In particular, the following medicines may increase the risk of side effects with XALKORI:
- Clarithromycin, telithromycin, erythromycin, antibiotics used to treat bacterial infections.
- Ketoconazole, itraconazole, posaconazole, voriconazole, used to treat fungal infections.
- Atazanavir, ritonavir, cobicistat, used to treat HIV/AIDS.
The following medicines may decrease the effectiveness of XALKORI:
- Phenytoin, carbamazepine, or phenobarbital, antiepileptics used to treat seizures or epilepsy.
- Rifabutin, rifampicin, used to treat tuberculosis.
- St. John's Wort (Hypericum perforatum), a herbal medicine used to treat depression.
XALKORI may increase the side effects associated with the following medicines:
- Alfentanil and other short-acting opioids like fentanyl (painkillers used for surgical procedures).
- Quinidine, digoxin, disopyramide, amiodarone, sotalol, dofetilide, ibutilide, verapamil, diltiazem, used to treat heart diseases.
- Blood pressure medicines called beta-blockers, such as atenolol, propranolol, labetalol.
- Pimozide, used to treat mental illnesses.
- Metformin, used to treat diabetes.
- Procainamide, used to treat heart rhythm disorders.
- Cisapride, used to treat stomach diseases.
- Cyclosporine, sirolimus, and tacrolimus, used in transplant patients.
- Ergot alkaloids (e.g., ergotamine, dihydroergotamine) used to treat migraines.
- Dabigatran, an anticoagulant used to reduce blood clotting.
- Colchicine, used to treat gout.
- Pravastatin, used to lower cholesterol levels.
- Clonidine, guanfacine, used to treat high blood pressure.
- Mefloquine, used to prevent malaria.
- Pilocarpine, used to treat glaucoma (a serious eye disease).
- Anticholinesterases, used to restore muscle function.
- Antipsychotics, used to treat mental illnesses.
- Moxifloxacine, used to treat bacterial infections.
- Methadone, used to treat pain and opioid dependence.
- Bupropion, used to treat depression and to help stop smoking.
- Efavirenz, raltegravir, used to treat HIV infection.
- Irinotecan, a chemotherapy medicine used to treat colon and rectal cancer.
- Morphine, used to treat acute and cancer pain.
- Naloxone, used to treat opioid addiction and withdrawal.
These medicines should be avoidedduring treatment with XALKORI.
Oral contraceptives
If you are taking oral contraceptives while taking XALKORI, oral contraceptives may be ineffective.
Taking XALKORI with food and drinks
XALKORI can be taken after a meal or on an empty stomach. Do not mix the XALKORI granules with food. You should avoid drinking grapefruit juice or eating grapefruit while being treated with XALKORI, as they may change the amount of XALKORI in your body.
Sun protection
Avoid spending too much time in the sun. XALKORI may make your skin more sensitive to the sun (photosensitivity), and you may burn more easily. Use protective clothing and/or sunscreen that covers your skin to protect yourself against sunburn if you need to be exposed to the sun during treatment with XALKORI.
Pregnancy and breastfeeding
If you are pregnant, may become pregnant, or are breastfeeding, consult your doctor or pharmacist before taking this medicine.
Women are advised to avoid becoming pregnant and men should not father a child during treatment with XALKORI, as this medicine may harm the fetus. An adequate method of contraception should be used during treatment and for at least 90 days after completing treatment, if there is any possibility that the person using this medicine may become pregnant or conceive a child, as oral contraceptives may be ineffective while taking XALKORI.
Do not breastfeed during treatment with XALKORI. XALKORI may harm the breastfed baby.
If you are pregnant, breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Be careful when driving or using machines, as patients treated with XALKORI may experience visual disturbances, dizziness, and fatigue.
XALKORI contains sucrose
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
3. How to take XALKORI granules in capsules for opening
Follow exactly the instructions for taking this medicine given by your doctor. If you are unsure, consult your doctor or pharmacist again.
- The recommended dose for children and adolescents with ALK-positive ALCL or ALK-positive IMT is 280 mg/m2 orally twice daily. The recommended dose will be calculated by the child's doctor and will depend on the child's weight and height (body surface area, BSA). The maximum daily dose in children and adolescents should not exceed 1000 mg. XALKORI should be given under the supervision of an adult.
- Give the recommended dose once in the morning and once in the evening.
- Give the capsules at approximately the same times each day.
- The granules should be given in the mouth and should not be crushed, chewed, or mixed with food.
- Do not swallow the capsule shell.
- Method of administration
For detailed instructions on the administration of XALKORI granules, see section 7 "Instructions for use" at the end of this leaflet.
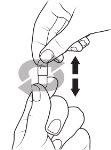
- Hold the capsule so that the "Pfizer" text is at the top, and tap the capsule to make all the granules fall to the bottom half of the capsule.
- Gently press the bottom of the capsule.
- Twist and remove the cap of the capsule.
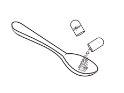
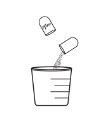
- Pour the granules directly into the child's mouth, OR pour the granules into a spoon or a measuring cup and then pour them into the child's mouth.
- Tap the opened capsule to make sure all the granules have been given.
- If you cannot give the full dose at one time, divide it into portions until you have given the full dose.
- Immediately after administration, give the child a glass of water to make sure they have swallowed all the granules.
- Once the child has swallowed the granules, you can give them other liquids or foods, except grapefruit and grapefruit juice.
If necessary, your doctor may reduce the dose to be taken orally. Your doctor may decide to permanently stop treatment with XALKORI if you cannot tolerate XALKORI.
If you take more XALKORI than you should
If you accidentally take more capsules, consult your doctor or pharmacist immediately. You may need medical attention.
If you forget to take XALKORI
The way to proceed if you forget to take a capsule depends on how long it is until your next dose:
- If your next dose is 6hours or moreaway, take the missed capsule as soon as possible. Then take the next capsule at the usual time.
- If your next dose is in less than 6hours, do not take the missed capsule. Take the next capsule at the usual time.
Tell your doctor about the missed dose at your next visit.
Do not take a double dose to make up for the missed capsule.
If you vomit after taking a dose of XALKORI, do not take an additional dose; take the next dose at the usual time.
If you stop taking XALKORI
It is important that you take XALKORI every day for as long as your doctor has prescribed it for you. If you are not able to take this medicine as your doctor has prescribed, or if you think you no longer need it, contact your doctor immediately.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If you experience adverse effects, consult your doctor, pharmacist, or nurse, even if they are adverse effects that do not appear in this leaflet.
Although not all adverse effects identified in adults with CPNM have been observed in children and adolescents with LACG or TMI, the same adverse effects should be considered for adult patients with lung cancer and for children and adolescents with LACG or TMI.
Some adverse effects can be serious. You should contact your doctor immediately if you experience any of the following serious adverse effects (see also section 2 "What you need to know before taking XALKORI"):
- Hepatic Insufficiency
Consult your doctor immediately if you feel more tired than usual, if your skin and the white areas of your eyes turn yellow, if your urine turns dark or brown (tea color), if you have nausea, vomiting, or less appetite, if you have pain in the right side of your stomach, or if you have itching or if you bruise more easily than normal. Your doctor may perform blood tests to check your liver function, and if the results of these tests are abnormal, they may reduce the dose of XALKORI or suspend treatment.
- Pulmonary Inflammation
Consult your doctor immediately if you experience difficulty breathing, especially if it is associated with coughing or fever.
- Reduction in the Number of White Blood Cells (including Neutrophils)
Consult your doctor immediately if you experience fever or infection. Your doctor may perform blood tests, and if the results are abnormal, your doctor may decide to reduce the dose of XALKORI.
- Feeling of Dizziness, Fainting, or Chest Pain
Consult your doctor immediately if you experience any of these symptoms, as they may be signs of changes in heart activity (observed in an electrocardiogram) or an abnormal heart rhythm. Your doctor may perform electrocardiograms to check that there are no problems with your heart during treatment with XALKORI.
- Partial or Complete Loss of Vision in One or Both Eyes
Consult your doctor immediately if you experience new visual problems, loss of vision, or any change in vision, such as difficulty seeing with one or both eyes. Your doctor may suspend or permanently interrupt treatment with XALKORI and refer you to an ophthalmologist.
For children and adolescents who receive XALKORI for the treatment of ALK-positive LACG or TMI: your doctor should refer you to an ophthalmologist before starting treatment with XALKORI, and within 1 month after starting treatment with XALKORI to detect visual problems. An ophthalmological examination should be performed every 3 months during treatment with XALKORI and more often if there are new visual problems.
- Severe Gastrointestinal Problems in Children and Adolescents with ALK-positive LACG or TMI
XALKORI may cause severe diarrhea, nausea, or vomiting. Inform your doctor immediately if you experience problems swallowing, vomiting, or diarrhea during treatment with XALKORI. Your doctor may give you medications as needed to prevent or treat diarrhea, nausea, and vomiting. Your doctor may recommend drinking more liquids or prescribing electrolyte supplements or other types of nutritional support if severe symptoms occur.
Other Adverse Effects of XALKORI Observed in Adults with CPNM May Include:
Very Common Adverse Effects(may affect more than 1 in 10 people)
- Visual effects (seeing flashes of light, blurred vision, sensitivity to light, seeing spots or double vision, usually appearing soon after starting treatment with XALKORI).
- Gastrointestinal problems, including vomiting, diarrhea, nausea.
- Edema (excess fluid in the body tissue that causes inflammation of the hands and feet).
- Constipation.
- Anomalies in liver tests in blood tests.
- Decreased appetite.
- Fatigue.
- Dizziness.
- Neuropathies (feeling of numbness or tingling in the joints or extremities).
- Alteration of the sense of taste.
- Abdominal pain.
- Reduction in the number of red blood cells in the blood (anemia).
- Rash.
- Reduced heart rate.
Common Adverse Effects(may affect up to 1 in 10 people)
- Indigestion.
- Increased levels of creatinine in the blood (may indicate that the kidneys are not functioning properly).
- Increased levels of the enzyme alkaline phosphatase in the blood (indicator of dysfunction or injury to an organ, especially the liver, pancreas, bones, thyroid gland, or gallbladder).
- Hypophosphatemia (low levels of phosphate in the blood that can cause confusion or muscle weakness).
- Fluid encapsulated within the kidney (renal cysts).
- Fainting.
- Inflammation of the esophagus (swallowing tube).
- Decreased levels of testosterone, a male sex hormone.
- Heart failure.
Uncommon Adverse Effects(may affect up to 1 in 100 people)
- Perforation in the stomach or intestine.
- Sensitivity to sunlight (photosensitivity).
- Elevated results in blood tests to check for muscle damage (high levels of creatine phosphokinase).
Other Adverse Effects of XALKORI Observed in Children and Adolescents with ALK-positive LACG or TMI May Include:
Very Common Adverse Effects(may affect more than 1 in 10 people)
- Anomalies in liver tests in blood tests.
- Visual effects (seeing flashes of light, blurred vision, sensitivity to light, seeing spots or double vision, usually appearing soon after starting treatment with XALKORI).
- Abdominal pain.
- Increased levels of creatinine in the blood (may indicate that the kidneys are not functioning properly).
- Anemia (reduction in the number of red blood cells in the blood).
- Low platelet count in blood tests (may increase the risk of bleeding and bruising).
- Fatigue.
- Decreased appetite.
- Constipation.
- Edema (excess fluid in the body tissue that causes inflammation of the hands and feet).
- Increased levels of the enzyme alkaline phosphatase in the blood (indicator of dysfunction or injury to an organ, especially the liver, pancreas, bones, thyroid gland, or gallbladder).
- Neuropathy (feeling of numbness or tingling in the joints or extremities).
- Dizziness.
- Indigestion.
- Alteration of the sense of taste.
- Hypophosphatemia (low levels of phosphate in the blood that can cause confusion or muscle weakness).
Common Adverse Effects(may affect up to 1 in 10 people)
- Rash.
- Inflammation of the esophagus (swallowing tube).
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of XALKORI
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiration date that appears on the bottle and carton after "EXP". The expiration date is the last day of the month indicated.
- This medicine does not require special storage conditions.
- Do not use this medicine if it is damaged or shows signs of deterioration.
Medicines should not be thrown away through wastewater or household waste. The empty capsules of XALKORI (oral granule) can be thrown away in household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
XALKORI Composition
- The active ingredient of XALKORI is crizotinib.
XALKORI 20 mg granule in capsules for opening: each capsule contains 20 mg of crizotinib
XALKORI 50 mg granule in capsules for opening: each capsule contains 50 mg of crizotinib
XALKORI 150 mg granule in capsules for opening: each capsule contains 150 mg of crizotinib
- Other components are (see also section 2 "XALKORI contains sucrose"):
Granule content: stearic alcohol, poloxamer, sucrose, talc (E553b), hypromellose (E464), macrogol (E1521), glycerol monostearate (E471), medium-chain triglycerides.
Capsule: gelatin, titanium dioxide (E171), brilliant blue (E133) or black iron oxide (E172).
Printing ink: shellac (E904), propylene glycol (E1520), potassium hydroxide (E525), black iron oxide (E172).
Product Appearance and Package Contents
The XALKORI granule is white to off-white and is found in an opening capsule.
XALKORI 20 mg granule in capsules for opening are capsules with a light blue cap with the text "Pfizer" printed in black ink and a white body with the text "CRZ 20" printed in black ink.
XALKORI 50 mg granule in capsules for opening are capsules with a gray cap with the text "Pfizer" printed in black ink and a light gray body with the text "CRZ 50" printed in black ink.
XALKORI 150 mg granule in capsules for opening are capsules with a light blue cap with the text "Pfizer" printed in black ink and a light blue body with the text "CRZ 150" printed in black ink.
It is available in plastic bottles with 60 opening capsules.
Marketing Authorization Holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Brussels
Belgium
Manufacturer
Pfizer Service Company BV
Hoge Wei 10
Zaventem
Vlaams-Brabant 1930
Belgium
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Spain
Pfizer, S.L.
Tel: +34 91 490 99 00
Date of the Last Revision of this Leaflet:08/2024.
Other Sources of Information
Detailed information about this medicine and information in different languages is available by scanning the QR code included below and on the box with a mobile device.
QR code to be included
Detailed information about this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
- Instructions for Use
Read the entire section 7 before using XALKORI granule in capsules for opening.
Items Needed for Administration of XALKORI:
- XALKORI granule in capsules, as prescribed by your doctor
- Optionally, a spoon or a measuring cup provided by the user
Preparation of XALKORI Granule (Steps 1 to 3):
Step 1 | Remove the required number of capsules for the prescribed dose of XALKORI granule from the corresponding bottle or bottles. |
Step 2 |
|
Step 3 | Hold the top and bottom parts of the capsule and turn them in opposite directions to separate them and open the capsule.
|
Administration of XALKORI Granule (Step 4):There are 2options for administering the oral granule to the child.
Step 4 | Option 1 (pouring the granule directly into the child's mouth) |
|
Option 2 (pouring the granule from an administration utensil) |
|
Once step 4 is completed, you can give the child other liquids or foods, except grapefruit and grapefruit juice.
Consult your doctor or pharmacist if you are not sure how to prepare or administer the prescribed dose of XALKORI granule to the child.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to XALKORI 50 mg CAPSULES, GRANULES FOR OPENINGDosage form: CAPSULE, 150 mgActive substance: crizotinibManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: CAPSULE, 20 mgActive substance: crizotinibManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: CAPSULE, 200mgActive substance: crizotinibManufacturer: Pfizer Europe Ma EeigPrescription required
Online doctors for XALKORI 50 mg CAPSULES, GRANULES FOR OPENING
Discuss questions about XALKORI 50 mg CAPSULES, GRANULES FOR OPENING, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





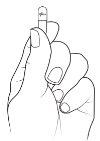 Tap the capsule gently to make all the granule fall to the bottom of the capsule. Carefully squeeze the bottom of the capsule so that the top comes loose.
Tap the capsule gently to make all the granule fall to the bottom of the capsule. Carefully squeeze the bottom of the capsule so that the top comes loose.