VIRAMUNE 400 mg PROLONGED-RELEASE TABLETS

How to use VIRAMUNE 400 mg PROLONGED-RELEASE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
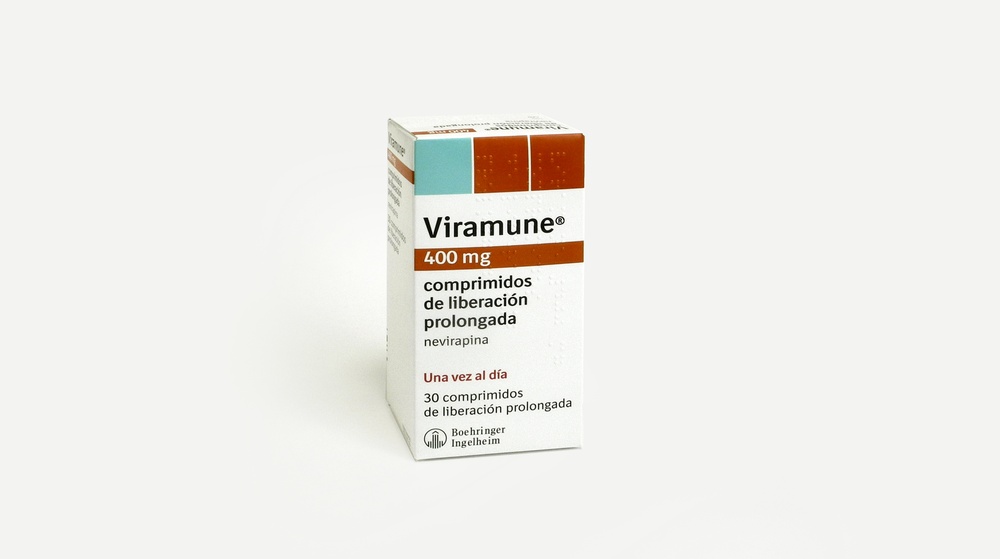
Viramune 400mg prolonged-release tablets
nevirapine
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Viramune and what is it used for
- What you need to know before you take Viramune
- How to take Viramune
- Possible side effects
- Storing Viramune
- Contents of the pack and other information
1. What is Viramune and what is it used for
Viramune belongs to a group of medicines called antiretrovirals, which are used to treat HIV infection.
The active substance in this medicine is nevirapine. Nevirapine belongs to a class of anti-HIV medicines called non-nucleoside reverse transcriptase inhibitors (NNRTIs). Reverse transcriptase is an enzyme that HIV needs to multiply. Nevirapine stops this enzyme from working. By stopping this enzyme from working, Viramune helps to control HIV-1 infection.
Viramune is used in the treatment of adults, adolescents, and children aged 3 years and above who are infected with HIV-1. You must take Viramune with other antiretroviral medicines. Your doctor will prescribe the most suitable medicines for you.
Viramune prolonged-release tablets should only be used after 2 weeks of treatment with another type of Viramune (suspension or immediate-release tablets), unless you are already being treated with Viramune and are switching to the prolonged-release form.
2. What you need to know before you take Viramune
Do not take Viramune
- if you are allergic to nevirapine or any of the other ingredients of this medicine (listed in section 6)
- if you have previously taken Viramune and had to stop due to:
- severe skin rash
- skin rash with other symptoms such as:
- fever
- blistering
- mouth sores
- eye inflammation
- facial swelling
- general swelling
- difficulty breathing
- muscle or joint pain
- general feeling of being unwell
- abdominal pain
- allergic reactions
- liver inflammation (hepatitis)
- if you have severe liver disease
- if you have had to stop treatment with Viramune in the past due to changes in your liver function
- if you are taking any medicines containing St. John's Wort (Hypericum perforatum). This product may cause Viramune to stop working properly.
Warnings and precautions
Talk to your doctor or pharmacist before you start taking Viramune.
During the first 18 weeks of treatment with Viramune, it is very important that you and your doctor monitor for signs of liver or skin reactions. These reactions can be severe and even life-threatening. The risk of these reactions is higher during the first 6 weeks of treatment.
If you experience a severe rash or allergic reaction (which may appear as a rash) along with other side effects such as
STOP TAKING VIRAMUNE AND CONTACT your doctor IMMEDIATELY, as these reactions can be life-threatening or cause death. If you experience only mild rash symptoms without any other reaction, inform your doctor immediately, who will advise you whether to stop taking Viramune. If you experience symptoms suggesting liver damage, such as
stop taking Viramune and contact your doctor immediately. If you experience severe liver, skin, or allergic reactions while taking Viramune, DO NOT TAKE VIRAMUNE AGAIN without first consulting your doctor. You must take your Viramune dose as directed by your doctor. This is especially important during the first 14 days of treatment (see more information in “How to take Viramune”). |
The following patients are at higher risk of developing liver problems:
- women
- patients infected with hepatitis B or C
- abnormal liver function tests
- patients who are naive to treatment with higher CD4 cell counts at the start of treatment with Viramune (women with more than 250 cells/mm³, men with more than 400 cells/mm³)
- pre-treated patients with detectable HIV-1 viral load in plasma and higher CD4 cell counts at the start of treatment with Viramune (women with more than 250 cells/mm³, men with more than 400 cells/mm³)
In some patients with advanced HIV infection (AIDS) and a history of opportunistic infections (AIDS-defining illnesses), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started. It is believed that these symptoms are due to an improvement in the body's immune response, enabling it to fight off infections that were present but not causing symptoms. If you notice any symptoms of infection, please inform your doctor immediately.
In addition to opportunistic infections, autoimmune disorders (a condition where the immune system attacks healthy body tissue) may also occur after you have started taking medicines for the treatment of your HIV infection. Autoimmune disorders may occur many months after the start of treatment. If you notice any symptoms of infection or other symptoms such as muscle weakness, weakness starting in the hands and feet and moving up towards the trunk of the body, palpitations, tremor, or hyperactivity, inform your doctor immediately to receive the necessary treatment.
Changes in body fat may occur in patients who are taking combined antiretroviral therapy. Talk to your doctor if you notice changes in body fat (see section 4 “Possible side effects”).
In some patients who are taking combined antiretroviral therapy, a bone disease called osteonecrosis (death of bone tissue due to loss of blood supply to the bone) may occur. The duration of combined antiretroviral therapy, the use of corticosteroids, alcohol consumption, severe immune system weakness, and high body mass index may be some of the many risk factors for developing this disease. The symptoms of osteonecrosis are: joint stiffness, pain, and discomfort, especially in the hip, knee, and shoulder, and difficulty moving. If you notice any of these symptoms, inform your doctor.
If you are taking nevirapine and zidovudine together, inform your doctor because you may need to have your white blood cell count checked.
Do not take Viramune after exposure to HIV unless you have been diagnosed with HIV and your doctor has told you to do so.
Prednisone should not be used to treat rashes associated with Viramune.
If you are taking oral contraceptives (e.g., “the pill”) or other hormonal methods of birth control while taking Viramune, you should also use a barrier method of contraception (e.g., condoms) to prevent pregnancy and transmission of HIV.
If you are receiving hormone replacement therapy, talk to your doctor before taking this medicine.
If you are taking or are prescribed rifampicin to treat tuberculosis, inform your doctor before taking this medicine with Viramune.
The prolonged-release tablets of Viramune or parts of the tablets may be eliminated and seen in the feces from time to time. These may look like whole tablets, but it has been shown that this does not affect the efficacy of nevirapine.
Children and adolescents
Viramune 400 mg prolonged-release tablets can be used in children if:
- they are 8 years of age or older and weigh 43.8 kg or more
- they are over 3 years of age and under 8 years of age and weigh 25 kg or more
- they have a body surface area of 1.17 square meters or above
For younger children, a liquid form of Viramune is available as an oral suspension.
Other medicines and Viramune
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Before you start taking Viramune, tell your doctor about all the other medicines you are taking. Your doctor may need to check that your other medicines are still working and make dose adjustments. Read the package leaflet carefully for all your other anti-HIV medicines that you take with Viramune.
It is especially important that you tell your doctor if you are taking or have recently taken:
- St. John's Wort (Hypericum perforatum, a herbal remedy for depression)
- rifampicin (a medicine for the treatment of tuberculosis)
- rifabutin (a medicine for the treatment of tuberculosis)
- macrolides, e.g., clarithromycin (a medicine for the treatment of bacterial infections)
- fluconazole (a medicine for the treatment of fungal infections)
- ketoconazole (a medicine for the treatment of fungal infections)
- itraconazole (a medicine for the treatment of fungal infections)
- methadone (a medicine used to treat opioid addiction)
- warfarin (a medicine to prevent blood clotting)
- hormonal contraceptives (e.g., “the pill”)
- atazanavir (another medicine for the treatment of HIV infection)
- lopinavir/ritonavir (another medicine for the treatment of HIV infection)
- fosamprenavir (another medicine for the treatment of HIV infection)
- efavirenz (another medicine for the treatment of HIV infection)
- etravirine (another medicine for the treatment of HIV infection)
- rilpivirine (another medicine for the treatment of HIV infection)
- zidovudine (another medicine for the treatment of HIV infection)
- elvitegravir/cobicistat (another medicine for the treatment of HIV infection)
Your doctor will carefully monitor the effect of Viramune and any of these medicines if you are taking them at the same time.
Taking Viramune with food and drink
There are no restrictions on taking Viramune with food and drink.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
It is not recommendedthat HIV-infected women breastfeed their babies because HIV can be transmitted through breast milk.
If you are breastfeeding or think you may want to breastfeed, you must askyour doctor as soon as possible.
Driving and using machines
When you take Viramune, you may experience fatigue. Be careful when taking part in activities such as driving or using tools or machines. If you experience fatigue, you should avoid potentially hazardous tasks such as driving and using tools or machines.
Viramune contains lactose
The prolonged-release tablets of Viramune contain lactose (milk sugar).
If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
3. How to take Viramune
Do not take Viramune on your own. You must take it with at least two other antiretroviral medicines. Your doctor will recommend the most suitable medicines for you.
Follow the instructions for taking this medicine exactly as your doctor has told you. If you are unsure, ask your doctor or pharmacist again.
Dose:
Adults
The dose is one 200 mg Viramune tablet per day for the first 14 days of treatment (the “lead-in” period). A separate pack of Viramune 200 mg tablets is available for this lead-in period. After 14 days, the usual dose is one 400 mg prolonged-release tablet once a day.
It is very important that you take only one Viramune tablet per day during the first 14 days (the “lead-in” period). If you experience a rash during this period, do not start taking Viramune 400 mg prolonged-release tablets and consult your doctor.
The 14-day lead-in period has been shown to reduce the risk of skin rash.
Patients who are already being treated with immediate-release tablets or oral suspension can be switched to prolonged-release tablets without the lead-in period.
As Viramune must always be taken in combination with other antiretroviral medicines, you must follow the instructions for your other medicines carefully. These are provided in the package leaflets for those medicines.
Viramune is also available as an oral suspension (for all age groups, weights, and body surface areas).
You must keep taking Viramune for as long as your doctor tells you.
As explained earlier in the section ‘Warnings and precautions’, your doctor will monitor you with liver function tests and check for signs of side effects such as rash. Depending on the results, your doctor may decide to stop or interrupt treatment with Viramune. Your doctor may also decide to restart treatment at a lower dose.
If you have kidney or liver problems of any degree, only take Viramune 200 mg tablets or Viramune 50 mg/5 ml oral suspension.
Only take Viramune prolonged-release tablets by mouth. Do not chew the prolonged-release tablets. You can take Viramune with or without food.
If you take more Viramune than you should
Do not take more Viramune than your doctor has prescribed and as described in this package leaflet. There is currently little information on the effects of an overdose of Viramune. If you have taken more Viramune than you should, talk to your doctor.
If you forget to take Viramune
Try not to miss any dose. If you realize you have missed a dose within 12 hours of the scheduled time, take the missed dose as soon as possible. If more than 12 hours have passed since the scheduled time, only take the next dose at the usual time.
If you stop taking Viramune
Taking your doses at the right times:
- greatly increases the effectiveness of your antiretroviral therapy
- reduces the chances of your HIV infection becoming resistant to antiretroviral medicines
It is important that you continue to take Viramune correctly, as described above, unless your doctor tells you to stop.
If you stop taking Viramune for more than 7 days, your doctor will advise you to start again with the 14-day lead-in period with Viramune tablets (as described above) before going back to taking one 400 mg prolonged-release tablet per day.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
During HIV treatment, there may be an increase in body weight and levels of glucose and lipids in the blood. This may be partly related to the recovery of health and lifestyle, and in the case of increased lipids in the blood, it may sometimes be due to HIV medications themselves. Your doctor will monitor these changes.
Like all medications, this medication may produce adverse effects, although not all people suffer from them.
As already mentioned in 'Warnings and Precautions',the most important adverse effects of Viramune are severe skin reactions and life-threatening liver damage. These reactions occur mainly during the first 18 weeks of treatment with Viramune. This is, therefore, an important period that requires close monitoring by your doctor.
If you observe any symptoms of a rash, inform your doctor immediately.
When a rash occurs, it is usually mild to moderate. However, in some patients, a rash appears in the form of a blistering skin reaction that can be severe or life-threatening (Stevens-Johnson syndrome and toxic epidermal necrolysis), and there have been reported fatalities. Most cases of rash, both severe and mild/moderate, occur during the first six weeks of treatment.
If a rash appears and you also feel unwell, you should interrupt treatment and go to your doctor immediately.
Hypersensitivity reactions (allergies) can occur. Such reactions can appear in the form of anaphylaxis (a severe allergic reaction) with symptoms such as:
- rash
- swelling of the face
- difficulty breathing (bronchospasm)
- anaphylactic shock
Hypersensitivity reactions can also be presented as a rash with other adverse effects such as:
- fever
- blistering of the skin
- sores in the mouth
- eye inflammation
- swelling of the face
- general swelling
- difficulty breathing
- muscle or joint pain
- decrease in the number of white blood cells in the blood (granulocytopenia)
- general malaise
- severe liver or kidney problems (liver or kidney failure)
If you experience a rash and any of the other adverse effects of a hypersensitivity reaction (allergy), inform your doctor immediately. These reactions can be fatal.
Abnormalities in liver function have been described with the use of Viramune. This includes some cases of liver inflammation (hepatitis), which can be sudden and severe (fulminant hepatitis) and liver failure, both of which can be fatal.
Inform your doctor if you experience any of the following clinical symptoms of liver damage:
- loss of appetite
- general malaise (nausea)
- vomiting
- yellowing of the skin (jaundice)
- abdominal pain
The following adverse effects have been reported in patients who received Viramune 200 mg tablets during the initial 14-day phase:
Frequent (may affect up to 1 in 10 people):
- rash
- fever
- headache
- abdominal pain
- general malaise (nausea)
- diarrhea
- feeling of fatigue
Uncommon (may affect up to 1 in 100 people):
- allergic reactions (hypersensitivity)
- allergic reactions characterized by rash, face inflammation, difficulty breathing (bronchospasm) or anaphylactic shock
- drug reaction with systemic symptoms (drug reaction with eosinophilia and systemic symptoms)
- severe and sudden liver inflammation (fulminant hepatitis)
- severe and potentially fatal skin rashes (Stevens-Johnson syndrome/toxic epidermal necrolysis)
- yellowing of the skin (jaundice)
- hives
- fluid under the skin (angioedema)
- muscle pain (myalgia)
- joint pain (arthralgia)
- decrease in blood phosphorus
- increase in blood pressure
Rare (may affect up to 1 in 1,000 people):
- liver inflammation (hepatitis)
- decrease in the number of red blood cells (anemia)
The following adverse effects have been reported in patients who received Viramune prolonged-release tablets once daily in the maintenance phase:
Frequent (may affect up to 1 in 10 people):
- rash
- headache
- abdominal pain
- general malaise (nausea)
- liver inflammation (hepatitis)
- feeling of fatigue
- abnormalities in liver function tests
- fever
- vomiting
- diarrhea
Uncommon (may affect up to 1 in 100 people):
- allergic reactions (hypersensitivity)
- allergic reactions characterized by rash, face inflammation, difficulty breathing (bronchospasm) or anaphylactic shock
- drug reaction with systemic symptoms (drug reaction with eosinophilia and systemic symptoms)
- severe and sudden liver inflammation (fulminant hepatitis)
- severe and potentially fatal skin rashes (Stevens-Johnson syndrome/toxic epidermal necrolysis)
- decrease in the number of red blood cells (anemia)
- decrease in the number of white blood cells (granulocytopenia)
- yellowing of the skin (jaundice)
- hives
- fluid under the skin (angioedema)
- muscle pain (myalgia)
- joint pain (arthralgia)
- decrease in blood phosphorus
- increase in blood pressure
When Viramune has been used in combination with other antiretroviral medications, the following events have also been reported:
- decrease in the number of red or platelet cells
- pancreatitis
- decrease or abnormalities in skin sensations
These effects are generally associated with other antiretroviral agents and may occur when Viramune is used in combination with other agents; however, it is unlikely that these effects are due to treatment with Viramune.
Other Adverse Effects in Children and Adolescents
A decrease in white blood cells (granulocytopenia) may occur, more frequently in children. A decrease in red blood cells (anemia), which may be related to treatment with nevirapine, is also more frequent in children. As with rash symptoms, inform your doctor of any adverse effect.
Reporting of Adverse Effects
If you experience any adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can help provide more information on the safety of this medication.
5. Storage of Viramune
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the packaging and on the blister or bottle after "EXP". The expiration date is the last day of the month indicated.
Viramune should be used within 2 months of opening the bottle.
This medication does not require special storage conditions.
Medications should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Viramune
- The active ingredient is nevirapine. Each prolonged-release tablet contains 400 mg of nevirapine.
- The other ingredients are lactose (in the form of monohydrate), hypromellose, yellow iron oxide, and magnesium stearate.
Appearance and Package Contents
Prolonged-release tablets are yellow, oval, and biconvex. The prolonged-release tablets measure approximately 9.3 × 19.1 mm, engraved with the code "V04" on one side and the company symbol on the other side. Viramune 400 mg prolonged-release tablets are available in blister packs of 30 or 90 prolonged-release tablets per box. Alternatively, Viramune 400 mg is available in bottles of 30 prolonged-release tablets. Not all pack sizes may be marketed.
Viramune is also available as an oral suspension or in the form of tablets.
Marketing Authorization Holder
Boehringer Ingelheim International GmbH
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
Manufacturer
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
or
Boehringer Ingelheim France
100-104 avenue de France
75013 Paris
France
You can obtain more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Boehringer Ingelheim Scomm Tél/Tel: +32 2 773 33 11 | Lietuva Boehringer Ingelheim RCV GmbH & Co KG Lietuvos filialas Tel.: +370 5 2595942 |
| Luxembourg/Luxemburg Boehringer Ingelheim Scomm Tél/Tel: +32 2 773 33 11 |
Ceská republika Boehringer Ingelheim spol. s r.o. Tel: +420 234 655 111 | Magyarország Boehringer Ingelheim RCV GmbH & Co KG Magyarországi Fióktelepe Tel.: +36 1 299 8900 |
Danmark Boehringer Ingelheim Danmark A/S Tlf: +45 39 15 88 88 | Malta Boehringer Ingelheim Ireland Ltd. Tel: +35 31 295 9620 |
Deutschland Boehringer Ingelheim Pharma GmbH & Co. KG Tel: +49 (0) 800 77 90 900 | Nederland Boehringer Ingelheim B.V. Tel: +31 (0) 800 22 55 889 |
Eesti Boehringer Ingelheim RCV GmbH & Co KG Eesti filiaal Tel: +372 612 8000 | Norge Boehringer Ingelheim Danmark Norwegian branch Tlf: +47 66 76 13 00 |
Ελλáδα Boehringer Ingelheim Ελλáς Μονοπρóσωπη Α.Ε. Tηλ: +30 2 10 89 06 300 | Österreich Boehringer Ingelheim RCV GmbH & Co KG Tel: +43 1 80 105-7870 |
España Boehringer Ingelheim España, S.A. Tel: +34 93 404 51 00 | Polska Boehringer Ingelheim Sp. z o.o. Tel.: +48 22 699 0 699 |
France Boehringer Ingelheim France S.A.S. Tél: +33 3 26 50 45 33 | Portugal Boehringer Ingelheim Portugal, Lda. Tel: +351 21 313 53 00 |
Hrvatska Boehringer Ingelheim Zagreb d.o.o. Tel: +385 1 2444 600 | România Boehringer Ingelheim RCV GmbH & Co KG Viena - Sucursala Bucuresti Tel: +40 21 302 2800 |
Ireland Boehringer Ingelheim Ireland Ltd. Tel: +353 1 295 9620 | Slovenija Boehringer Ingelheim RCV GmbH & Co KG Podružnica Ljubljana Tel: +386 1 586 40 00 |
Ísland Vistor ehf. Tel: +354 535 7000 | Slovenská republika Boehringer Ingelheim RCV GmbH & Co KG organizacná zložka Tel: +421 2 5810 1211 |
Italia Boehringer Ingelheim Italia S.p.A. Tel: +39 02 5355 1 | Suomi/Finland Boehringer Ingelheim Finland Ky Puh/Tel: +358 10 3102 800 |
Κúπρος Boehringer Ingelheim Ελλ?ς Μονοπρóσωπη Α.Ε. Tηλ: +30 2 10 89 06 300 | Sverige Boehringer Ingelheim AB Tel: +46 8 721 21 00 |
Latvija Boehringer Ingelheim RCV GmbH & Co KG Latvijas filiale Tel: +371 67 240 011 | United Kingdom (Northern Ireland) Boehringer Ingelheim Ireland Ltd. Tel: +353 1 295 9620 |
Date of Last Revision of this Leaflet:{MM/YYYY}
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VIRAMUNE 400 mg PROLONGED-RELEASE TABLETSDosage form: MODIFIED-RELEASE TABLET, 400 mgActive substance: nevirapineManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 200 mgActive substance: nevirapineManufacturer: Kern Pharma S.L.Prescription requiredDosage form: TABLET, 200 mgActive substance: nevirapineManufacturer: Laboratorios Normon S.A.Prescription required
Online doctors for VIRAMUNE 400 mg PROLONGED-RELEASE TABLETS
Discuss questions about VIRAMUNE 400 mg PROLONGED-RELEASE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions