
VARILRIX powder and solvent for injectable solution in pre-filled syringe

How to use VARILRIX powder and solvent for injectable solution in pre-filled syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Varilrix Powder and Solvent for Solution for Injection in Pre-filled Syringe
Varicella Vaccine (Live Virus)
Read all of this leaflet carefully before you or your child receive this medicine, because it contains important information for you/your child.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed to you or your child, and you should not give it to others.
- If you or your child experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Varilrix and what is it used for
- What you need to know before you or your child receive Varilrix
- How Varilrix is administered
- Possible side effects
- Storage of Varilrix
- Contents of the pack and further information
1. What is Varilrix and what is it used for
Varilrix is a vaccine for use in individuals from 12 months of age to protect them against varicella. In some circumstances, Varilrix can also be administered to infants from 9 months of age.
Vaccination within 3 days after contact with a case of varicella may help prevent varicella or reduce the severity of the disease.
How Varilrix works
When a person is vaccinated with Varilrix, the immune system (the body's natural defense system) will produce antibodies to protect the person from varicella virus infection. Varilrix contains weakened viruses, so it is very unlikely to cause varicella in healthy individuals.
As with any vaccine, Varilrix may not completely protect all vaccinated individuals.
2. What you need to know before you or your child receive Varilrix
Do not useVarilrix
- If you or your child have any disease (such as blood disorders, cancer, Human Immunodeficiency Virus (HIV) infection, or Acquired Immune Deficiency Syndrome (AIDS)) or are taking any medication (including high doses of corticosteroids) that may weaken the immune system. Whether you or your child receive the vaccine will depend on the level of your/their immune system. See section 2 "Warnings and precautions".
- If you or your child are allergic to any of the components of this vaccine (listed in section 6). Signs of an allergic reaction may include: skin rash with itching, difficulty breathing, and swelling of the face or tongue.
- If you or your child are allergic to neomycin (an antibiotic). A history of contact dermatitis (skin rash caused by direct contact with allergens such as neomycin) should not be a reason not to vaccinate. However, consult your doctor first.
- If you or your child have previously had an allergic reaction to any varicella vaccine.
- If you or your daughter are pregnant. Additionally, pregnancy should be avoided for 1 month after vaccination.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before you or your child receive Varilrix:
- If you or your child have a severe infection with high fever. Vaccination may need to be postponed until recovery. A minor infection, such as a cold, does not require postponing vaccination. However, consult your doctor first.
- If you or your child have a weakened immune system due to diseases (e.g., HIV infection) and/or treatments. You or your child should be closely monitored as the response to vaccination may not be sufficient to ensure protection against the disease (see section 2 "Do not use Varilrix").
- if you have bleeding problems or bruise easily.
Fainting (especially in adolescents) may occur after any injection, so you should inform your doctor or nurse if you or your child have fainted after previous injections.
As with any vaccine, Varilrix may not completely protect you or your child against varicella. However, individuals who have been vaccinated and develop varicella usually have a very mild disease compared to unvaccinated individuals.
Rarely, the weakened virus can be transmitted from a vaccinated person to others. This usually occurs when the vaccinated person has some spots or blisters on the skin. Healthy individuals who become infected in this way usually only develop a mild skin rash that is not harmful.
After vaccination, you or your child should avoid, as much as possible, close contact with the following individuals for 6 weeks after vaccination:
- individuals with weakened immune systems,
- pregnant women who have not had varicella or have not been vaccinated against varicella,
- newborn babies of mothers who have not had varicella or have not been vaccinated against varicella.
Other medicines and Varilrix
Tell your doctor or pharmacist if you or your child are taking, have recently taken, or might take any other vaccine and/or medicine.
Tell your doctor if you or your child need to have a skin test to detect tuberculosis. If this test is performed within 6 weeks after Varilrix administration, the result may not be reliable.
Vaccination should be delayed for at least 3 months if you or your child have received a blood transfusion or human antibodies (immunoglobulins).
Aspirin or other salicylates (a substance present in some medicines used to reduce fever and relieve pain) should be avoided for 6 weeks after Varilrix vaccination, as this can cause a serious disease called Reye's syndrome that can affect all organs of the body.
Varilrix can be administered at the same time as other vaccines. A different injection site will be used for each vaccine.
Pregnancy and breastfeeding
Varilrix should not be administered to pregnant women.
If you or your daughter are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before receiving the vaccine. It is also important that you or your daughter do not become pregnant for 1 month after vaccination. During this time, an effective contraceptive method should be used to avoid pregnancy.
Tell your doctor if you or your daughter are breastfeeding or plan to breastfeed. Your doctor will decide whether you or your daughter should receive Varilrix.
Driving and using machines
Varilrix has a negligible influence on the ability to drive and use machines. However, some of the effects mentioned in section 4 "Possible side effects" may temporarily affect the ability to drive or use machines.
Varilrix contains sorbitol and phenylalanine
This vaccine contains 6 mg of sorbitol in each dose unit.
This vaccine contains 331 micrograms of phenylalanine in each dose unit. Phenylalanine may be harmful in case of phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body cannot eliminate it properly.
3. How Varilrix is administered
Varilrix is injected under the skin or into a muscle, either in the upper arm or in the outer thigh.
Individuals from 12 months of age should receive 2 doses of Varilrix with at least 6 weeks between doses. The time between the first and second doses must notbe less than 4 weeks.
In some circumstances, the first dose of Varilrix can be administered to infants from 9 to 11 months of age. In these cases, two doses are needed and should be administered with at least 3 months between doses.
Individuals at risk of severe varicella, such as those receiving cancer treatment, may receive additional doses. The interval between doses must notbe less than 4 weeks.
Your doctor will determine the appropriate time and number of doses based on the relevant official recommendations.
If you or your child receive more Varilrix than you/they should
Overdose is very unlikely because the vaccine is provided in a single-dose vial and is administered by a doctor or nurse. However, if you or your child receive more Varilrix than you/they should, consult your doctor, pharmacist, or nurse immediately or call the Toxicology Information Service, phone 91 562 04 20.
A few cases of accidental administration have been reported, and in some of these cases, abnormal drowsiness and seizures (convulsions) were reported.
If you think you or your child have missed a dose of Varilrix
Contact your doctor, who will decide if a dose is needed and when to administer it.
4. Possible side effects
As with all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects can occur with this vaccine:
- Very common (may affect more than 1 in 10 people):
- pain and redness at the injection site
- Common (may affect up to 1 in 10 people):
- skin rash (spots and/or blisters)
- swelling at the injection site*
- fever of 38°C or higher (rectal)*
- Uncommon (may affect up to 1 in 100 people):
- upper respiratory tract infection
- sore throat and difficulty swallowing (pharyngitis)
- swelling of the lymph nodes
- irritability
- headache
- drowsiness
- cough
- itching, discharge, or congestion of the nose, sneezing (rhinitis)
- nausea
- vomiting
- rash similar to varicella
- itching
- joint pain
- muscle pain
- fever higher than 39.5°C (rectal)
- lack of energy (fatigue)
- general malaise
- Rare (may affect up to 1 in 1000 people):
- eye inflammation (conjunctivitis)
- stomach pain
- diarrhea
- hives (urticaria)
*Swelling at the injection site and fever may occur very commonly in adolescents and adults. Swelling may also occur very commonly after the second dose in children under 13 years of age.
The following side effects have been reported in some cases during routine use of Varilrix:
- shingles
- small hemorrhagic spots or easy bruising due to a decrease in a type of blood cells called platelets
- allergic reactions. Rashes that can cause itching or blisters, swelling of the eyes and face, difficulty breathing or swallowing, sudden drop in blood pressure, and loss of consciousness. These reactions may occur before leaving the doctor's office. However, if you or your child develop any of these symptoms, contact a doctor urgently.
- infection or inflammation of the brain, spinal cord, and peripheral nerves that can cause temporary difficulty walking (instability) and/or temporary loss of control of body movements, stroke (brain damage caused by interruption of blood flow)
- seizures or convulsions
- inflammation, narrowing, or blockage of blood vessels. This can involve unusual bleeding or bruising under the skin (Henoch-Schönlein purpura) or fever lasting more than five days associated with a rash on the trunk, followed by peeling of the skin on hands and fingers, redness of the eyes, lips, throat, and tongue (Kawasaki disease)
- erythema multiforme (symptoms are red spots, often with itching, similar to measles rash, starting on the extremities and sometimes on the face and the rest of the body).
Reporting of side effects
If you or your child experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's online platform, https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Varilrix
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date stated on the packaging. The expiry date is the last day of the month stated.
Store and transport in a refrigerator (between 2°C and 8°C).
Store in the original packaging to protect from light.
After reconstitution, the vaccine should be administered immediately.
If it is not possible, the reconstituted vaccine can be stored for up to 90 minutes at room temperature (25°C) or up to 8 hours in a refrigerator (between 2°C and 8°C). If the vaccine is not used within the recommended storage conditions, the reconstituted vaccine should be discarded.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicine to a pharmacy for proper disposal. If you have any questions, ask your pharmacist. This will help protect the environment.
6. Container contents and additional information
Composition of Varilrix
- The active principle is: live attenuated varicella virus (Oka strain, produced in
human diploid MRC-5 cells). Each 0.5 ml dose of the reconstituted vaccine contains no less than 10^3.3 UFP (plaque-forming units) of the varicella virus.
- The other components are:
Powder: amino acids (containing phenylalanine), anhydrous lactose, sorbitol (E-420), mannitol (E-421).
Solvent: water for injectable preparations.
Appearance of the product and container contents
Varilrix is presented as a powder and solvent for injectable solution (powder in a vial for 1 dose and solvent in a pre-filled syringe (0.5 ml), with or without separate needles in the following package sizes:
- with 1 separate needle: package sizes of 1 or 10.
- with 2 separate needles: package sizes of 1 or 10.
- without needles: package sizes of 1 or 10.
Varilrix is supplied as a light cream to yellowish or pinkish powder and a clear, colorless solvent (water for injectable preparations) to reconstitute the vaccine.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
GlaxoSmithKline, S.A.
PTM C/ Severo Ochoa 2
28760 - Tres Cantos
Madrid
Phone: 900 202 700
Fax: 91 807 03 10
e-mail: [email protected]
Manufacturer
GlaxoSmithKline Biologicals
Rue de l´Institut 89
1330 Rixensart (Belgium)
This medicinal product is authorized in the Member States of the European Economic Areawith the following names:
Member State | Name |
Germany, Austria, Belgium, Cyprus, Denmark, Estonia, Finland, France, Greece, Hungary, Iceland, Italy, Luxembourg, Malta, Norway, Poland, Portugal, Czech Republic, Romania, Sweden | VARILRIX |
Spain | VARILRIX powder and solvent for injectable solution in pre-filled syringe |
Latvia | Varilrix pulveris un škidinatajs injekciju škiduma pagatavošanai pilnšlirce |
Lithuania | Varilrix milteliai ir tirpiklis injekciniam tirpalui užpildytame švirkšte |
Date of the last revision of this leaflet:05/2025
Other sources of information
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
-------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
As with all injectable vaccines, adequate medical supervision should be available and medical treatment should be available in case of a rare anaphylactic reaction after administration of the vaccine.
The alcohol and other antiseptic agents should be allowed to evaporate from the skin before injecting the vaccine, as they may inactivate the attenuated viruses in the vaccine.
Varilrix should not be administered intravascularly or intradermally.
In the absence of compatibility studies, this medicinal product should not be mixed with others.
The solvent and the reconstituted vaccine should be visually inspected. The color of the reconstituted vaccine may vary between light orange and pink due to small variations in its pH. It may containtranslucent particles related to the product. This is normal and does not compromise the action of the vaccine.
Do not administer if the vaccine has a different color or contains other particles.
The vaccine should be reconstituted by adding the entire contents of the pre-filled syringe of solvent to the vial containing the powder.
To learn how to insert the needle into the syringe, read carefully the instructions provided with images 1 and 2. However, the syringe provided with Varilrix may be slightly different (without screw thread) than the syringe in the image. In this case, the needle should be inserted without screwing.
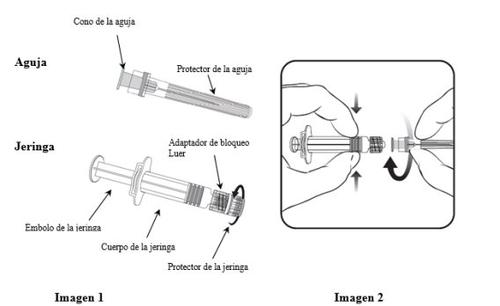
Always hold the syringe by the body, not by the plunger or the Luer lock adapter (LLA), and keep the needle in the axis of the syringe (as shown in image 2). Otherwise, the LLA could be deformed and cause leaks.
If the LLA comes off during syringe assembly, use a new dose of the vaccine (new syringe and vial).
- Unscrew the syringe protector by turning it counterclockwise (as shown in image 1).
Whether the LLA rotates or not, please follow the next steps:
- Insert the needle into the syringe by gently fitting the cone of the needle into the LLA and turning a quarter turn clockwise until it clicks (as shown in image 2).
- Remove the needle protector (it may be difficult).
- Add the solvent to the powder. The mixture should be shaken well until the powder is completely dissolved.
- Remove all the contents of the vial
- A new needle should be used to administer the vaccine. Unscrew the needle from the syringe and insert the needle for injection by repeating the previous step 2.
After reconstitution, it is recommended to inject the vaccine as soon as possible. However, it has been shown that the reconstituted vaccine can be stored for up to 90 minutes at room temperature (25°C) and up to 8 hours in the refrigerator (between 2°C and 8°C). If it is not used within the recommended use and storage conditions, the reconstituted vaccine should be discarded.
The disposal of the unused vaccine and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VARILRIX powder and solvent for injectable solution in pre-filled syringeDosage form: INJECTABLE, minimum of 1350 UFP/0.5 ml doseActive substance: varicella, live attenuatedManufacturer: Schering Plough S.A.Prescription requiredDosage form: INJECTABLE, 50 microgramsActive substance: zoster, purified antigenManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 60 micrograms/dose + 60 micrograms/doseActive substance: respiratory syncytial virus vaccinesManufacturer: Pfizer Europe Ma EeigPrescription required
Online doctors for VARILRIX powder and solvent for injectable solution in pre-filled syringe
Discuss questions about VARILRIX powder and solvent for injectable solution in pre-filled syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















