
TRUTEST 36 Adhesive Patch for Allergen Provocation Test

How to use TRUTEST 36 Adhesive Patch for Allergen Provocation Test
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
TRUTEST 36Adhesive Patch for Allergen Provocation Test
TRUTEST 36 consists of 3 strips of surgical tape with 12 patches each. 35 patches are coated with a film containing a specific allergen or mixture of allergens. One of the patches (patch No. 9) is empty.
Active Substance | micrograms/cm2 | micrograms/patch | |
Panel 1 |
| 200 | 162 |
| 1000 | 810 | |
| 600 | 486 | |
| 54 | 44 | |
| 630 | 510 | |
| 430 | 348 | |
| 1200 | 972 | |
| 1000 | 810 | |
| - | - | |
| 800 | 648 | |
| 50 | 41 | |
| 20 | 16 | |
Panel 2 |
| 45 | 36 |
| 50 | 41 | |
| 250 | 203 | |
| 75 | 61 | |
| 4 | 3 | |
| 100 | 81 | |
| 5.0 | 4.1 | |
| 80 | 65 | |
| 180 | 146 | |
| 75 | 61 | |
| 7 | 6 | |
| 27 | 22 | |
Panel 3 |
| 550 | 450 |
| 190 | 154 | |
| 3.0 | 2.4 | |
| 75 | 61 | |
| 600 | 490 | |
| 1.0 | 0.81 | |
| 20 | 16 | |
| 75 | 61 | |
| 600 | 490 | |
| 3.0 | 2.4 | |
| 50 | 41 | |
| 250 | 200 |
- Five parts of benzocaine, one part of cinchocaine hydrochloride, and tetracaine hydrochloride.
- Five parts of geraniol and oak moss, four parts of hydroxy citronellal and cinnamic alcohol, two parts of cinnamaldehyde and eugenol, and one part of isoeugenol and α-amylcinnamaldehyde.
- Equal weights of methylparaben, ethylparaben, propylparaben, butylparaben, and benzylparaben.
- Equal weights of diphenylguanidine, zinc diethyldithiocarbamate, and zinc dibutyldithiocarbamate.
- Two parts of N-isopropyl-N'-phenyl paraphenylenediamine, five parts of N-cyclohexyl-N'-phenyl paraphenylenediamine, and five parts of N,N'-diphenyl paraphenylenediamine.
- Contains N-hydroxymethyl succinimide.
- Equal parts of morpholinyl mercaptobenzothiazole, N-cyclohexylbenzothiazylsulfenamide, and dibenzothiazol disulfide.
- Equal parts of disulfiram, dipentamethylenethiuram disulfide, tetramethylthiuram disulfide, and tetramethylthiuram monosulfide.
- Equal weights of clioquinol and chlorquinaldol
Read the entire package leaflet carefully before starting to use this medicine,as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is TRUTEST 36 and what is it used for
- What you need to know before you start using TRUTEST 36
- How to use TRUTEST 36
- Possible side effects
- Storage of TRUTEST 36
- Package contents and further information
1. What is TRUTEST 36 and what is it used for
TRUTEST 36 is used to diagnose allergic contact dermatitis. Contact dermatitis is a skin reaction caused by exposure to foreign substances that trigger an allergic reaction.
TRUTEST 36 is a ready-to-use adhesive patch test to determine the cause of allergic contact dermatitis.
TRUTEST 36 is indicated for adults.
The test consists of 3 strips of surgical tape. The strips contain 12 patches each. Each patch is coated with a film containing a substance that can cause a skin reaction in sensitive individuals. These substances are called allergens. Each patch contains a different allergen, and one of the patches is empty. TRUTEST 36 contains 35 of the most common allergens/mixtures of allergens and one empty patch.
TRUTEST 36 shows whether you are allergic to any of the test substances (allergens) on the patches.
If a substance you are allergic to comes into contact with your skin, it causes an inflammatory reaction called contact dermatitis.
These substances could be an ingredient in your perfume or shaving lotion, a cream or ointment, rubber gloves, industrial chemicals, etc. The substances in TRUTEST 36 are known allergens. If you are allergic to the substance on a particular TRUTEST 36 patch, then the skin under the patch will react to that substance, becoming red and inflamed. If you are not allergic to a particular patch, the skin under the patch will not react. You can be allergic to more than one patch.
2. What you need to know before you start using TRUTEST 36
Do not use TRUTEST 36:
- If you have severe or widespread dermatitis. The test should be delayed until the acute phase of the dermatitis has ended.
- If you are allergic to any of the excipients of TRUTEST 36 (listed in section 6).
Warnings and precautions
- You should avoid exposing the test area to the sun. Sun tanning can hide positive reactions to the allergens you are actually allergic to.
- Avoid excessive sweating while wearing the test strips.
- If you are taking medications that suppress the immune system, such as steroids (e.g., prednisolone) or steroid creams/ointments (e.g., hydrocortisone). These medications should not be used for at least two weeks before the test.
- If you have a skin irritation syndrome. This is a state of increased irritability of the skin caused by a reaction in other parts of the body. If you react to all the patches, your doctor may need to repeat the test another day.
- If you have had anaphylactoid reactions before. The use of TRUTEST 36 should be carefully considered.
Talk to your doctor before using TRUTEST 36 if any of these factors apply to you. Your doctor will decide what to do.
Sensitization: In rare cases, you may develop sensitivity to a substance on the test patch while using TRUTEST 36. A test reaction that appears more than 10 days after application may be a sign of contact sensitization.
TRUTEST 36 should only be applied to the skin:
- without acne
- without scars
- without dermatitis
- in a condition that will not interfere with the test results. Consult your doctor if you have any doubts.
Moisture around the test should be avoided. Therefore, when bathing or showering, be careful not to get the test panel or its surroundings wet. If the test panel gets wet, it may come off, allowing water to wash away the test substances.
Avoid any activity, such as sunbathing or exercising, that may cause the patches to come off.
Butylhydroxyanisole (BHA) (E320) and butylhydroxytoluene (BHT) (E321) are present in the allergenic patch No. 7 Colophony (panel 1) for stability reasons. BHA and BHT may cause local skin reactions (e.g., contact dermatitis), which may result in a false positive reaction for Colophony.
Children
TRUTEST 36 is not recommended for use in children, as its safety and efficacy have not been established in these patients.
Other medicines and TRUTEST 36
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription, before applying TRUTEST 36. Remember that your specialist doctor needs to know if you are taking other medicines.
Since steroids can suppress a positive test reaction, the use of topical steroids in the application area or oral steroids equivalent to 20 mg of prednisolone or more daily should be discontinued at least two weeks before the test.
Pregnancy, breast-feeding, and fertility
Pregnant women should not use TRUTEST 36. It is important to inform your doctor if you are pregnant or think you may be pregnant.
You should not breast-feed your baby during the use of TRUTEST 36.
Driving and using machines
TRUTEST 36 is unlikely to affect your ability to drive or use machines. Consult your doctor if you have any doubts.
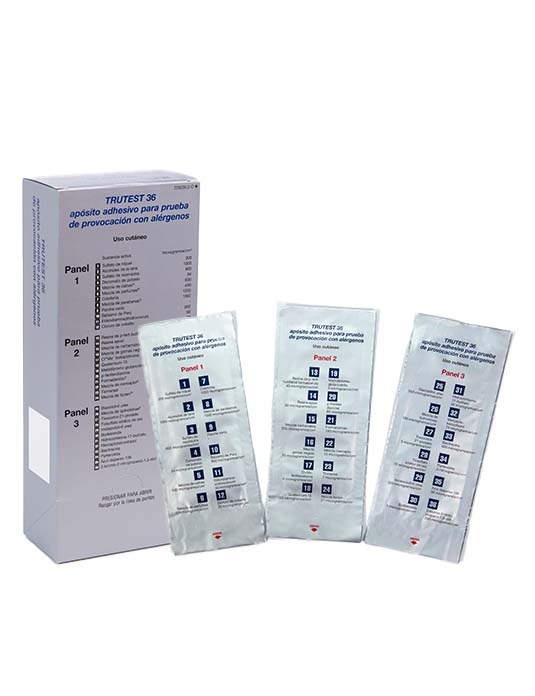
3. How to use TRUTEST 36
TRUTEST 36 should be applied by your doctor.
- Open the package and remove the TRUTEST 36 panel.
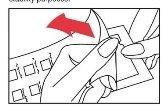
- Remove the protective plastic cover from the surface of the panel. Be careful not to touch the test substances. A desiccant is included in the package of panel 2 for stability reasons.
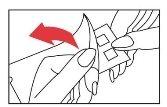
- Place the patch on the upper back area of the patient. However, it can also be placed on the outer aspect of the upper arm. Smooth the panel gently from the center outwards, ensuring that each allergen comes into contact with the skin properly. The two panels are best placed on either side of the spine, about a few centimeters apart.
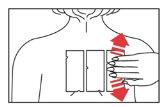
- Mark the two notches on the panels (top left and bottom edge) with a medical marker.
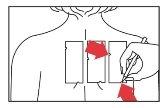
You should wear the test strips for 48 hours without removing them. You should be careful not to get the test area wet (water, sweat).
If the patch is removed or comes off too early, it may not produce positive reactions to the allergens that you are actually allergic to. Inform your doctor if the patch is removed or comes off before 48 hours.
After 48 hours, you or your doctor can remove the panels.
When should the results be read?
Your doctor will read the test result half an hour after removing the test and again 1 or 2 days after removing the test, when any allergic reaction is fully developed and any irritant reactions have disappeared. Some allergens may sometimes cause reactions that do not appear until 4-5 days after removing the test. Please inform your doctor if this happens.
What should the doctor look for?
The doctor will carefully examine the test area for signs of an allergic reaction. This reaction usually consists of a rash with swelling, redness, and small blisters. However, redness alone does not necessarily mean it is an allergic reaction. If you are allergic, your doctor will provide you with the following information:
- In what part of your daily environment you are likely to come into contact with the harmful substances.
- The best way to avoid these substances. Your doctor may suggest alternatives to the items you should avoid.
If you have any doubts, consult your doctor or pharmacist.
Contact your doctor if you experience severe discomfort in the area where the test was applied. Your doctor will decide whether to remove the patches.
In case of accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common side effects (may affect more than 1 in 10 people):
- Irritation caused by the surgical tape may occur, but it usually disappears quickly.
- Burning sensation.
- Long-lasting reactions. A positive test reaction usually disappears within 1-2 weeks, while a long-lasting reaction may persist for weeks or months.
Common side effects (may affect up to 1 in 10 people):
- The test reactions may leave an area of lighter or darker skin color temporarily.
- Redness of the skin caused by irritation or inflammation (erythema)
Uncommon side effects (may affect up to 1 in 100 people):
- A flare-up of dermatitis.
Rare side effects (may affect up to 1 in 1,000 people):
- Sensitization to a substance on the test patch may occur.
Frequency not known (cannot be estimated from the available data):
- Anaphylactic reaction (systemic reaction, possibly with a drop in blood pressure that can be life-threatening).
- Hypersensitivity (allergic reaction).
In extremely rare cases and only in relation to certain substances, anaphylactic reactions (systemic reaction, possibly with a drop in blood pressure that can be life-threatening) have occurred. Allergy departments are prepared for other reasons to treat these incidents. Anaphylactoid reactions related to the application of TRUTEST 36 are not documented.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of TRUTEST 36
Keep in a refrigerator (between 2°C and 8°C).
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the package after “EXP:”. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the SIGRE collection point at your pharmacy. If you have any doubts, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Contents and Additional Information
Composition of TRUTEST 36
- In addition to the active substances indicated on the first page, the test contains the following excipients: Polyester fiber tape with adhesive (ethylene-vinyl acetate copolymer) with acrylic adhesive, polyester patches, povidone 90, hydroxypropylcellulose, methylcellulose, ß-cyclodextrin, sodium carbonate, sodium bicarbonate, butylhydroxyanisole, and butylhydroxytoluene.
Appearance of the Product and Container Contents
Each panel is coated with a protective layer of silicone-coated polyethylene and packaged in hermetically sealed laminated bags.
The laminated bag of Panel 2 also contains a desiccant to properly preserve the allergens during storage.
Container contents: 10 tests (1 test = one Panel 1, one Panel 2, and one Panel 3)
Marketing Authorization Holder and Manufacturer
SmartPractice Denmark ApS
Herredsvejen 2
3400 Hillerød
Denmark
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
MARTI TOR ALERGIA, S.L.
C/Ull de Llebre, 16
08758 Cervelló (Barcelona)
Spain
Date of the last revision of thisleaflet:February 2021
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
This information is intended solely for healthcare professionals:
With each package of TRUTEST 36, an identification template is provided for rapid identification of any allergen that causes a reaction. To ensure correct positioning, the marks on the skin must correspond to the slots on the template. The difference between page 1 and 2 of the template, which correspond to panel 1 and panel 2 respectively, should be taken into account.
The recommended interpretation method by the International Contact Dermatitis Research Group is:
- | Negative reaction |
? | Doubtful reaction: weak macular erythema, no or insignificant infiltration |
+ | Weak positive reaction (non-vesicular): erythema, weak infiltration, possible papules |
++ | Strong positive reaction (vesicular): erythema, infiltration, papules, vesicles |
+++ | Extreme positive reaction: intense erythema, infiltration, coalescing vesicles |
RI | Irritative reaction of different types |
NA | Not analyzed |
Note
- Patients showing a negative reaction may still be sensitive to another substance not included in this test panel. Furthermore, false-negative results may occur. The test may need to be repeated or performed with complementary substances.
- A positive reaction must meet the criteria for an allergic reaction (papular or vesicular erythema and infiltration).
- Pustules, as well as homogeneous or irregular follicular erythema without infiltration, are usually signs of irritation and do not indicate allergy.
What is important in the evaluation of a positive response is not the number of positives assigned to the test response, but the determination of whether the response is a truly positive reaction (caused by allergy) or a non-specific irritative reaction.
Some of the allergens (neomycin sulfate, p-phenylenediamine, wool alcohols, mixture of caines, gold sodium thiosulfate, parthenolide, dispersed blue 106, bacitracin, imidazolidinyl urea, diazolidinyl urea, budesonide, hydrocortisone-17-butyrate, and tixocortol-21-pivalate) may sometimes cause reactions that may not appear until 4-5 days after application. Patients should be warned to inform their doctor if this reaction appears. An additional medical review between the 5th and 7th day will verify a late reaction if necessary.
All positive reactions should be carefully evaluated, considering the individual patient's clinical history and symptoms, especially in the case of positive reactions to specific allergens with lower sensitization rates (e.g., gold sodium thiosulfate).
Contraindications
Severe or generalized dermatitis. The test should be postponed until the acute phase has ended.
Known hypersensitivity to any of the excipients included in the test in addition to the active principles.
Special Warnings and Precautions for Use
The substances in the test panel rarely produce sensitization. A reaction that appears on the 10th day or later may be a sign of contact sensitization.
The skin irritation syndrome is a state of hyperreactivity induced by dermatitis in other parts of the body or by a strong positive reaction to the test. Therefore, the results should be carefully evaluated in patients with multiple, concomitant positive test results. It may be necessary to repeat the test at a later date to determine which reactions are false positives.
Before application, the use of TRUTEST 36 should be cautiously evaluated in patients with a history of anaphylactoid reactions.
Excessive sweating and sun exposure of the test area should be avoided. Solar tanning can decrease the reactivity of the patches and cause false negatives.
Application of the test on skin with acne, scars, dermatitis, or any other condition that may interfere with the results should be avoided.
If a severe reaction occurs due to the test, the patient may be treated with a topical corticosteroid or, in rare cases, with a systemic corticosteroid.
Butylhydroxyanisole (BHA) (E320) and butylhydroxytoluene (BHT) (E312) are present as antioxidants in allergenic patch no. 7 Colophony (panel 1). BHA and BHT may cause local skin reactions (e.g., contact dermatitis), which may result in a false positive reaction for Colophony.
- Country of registration
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRUTEST 36 Adhesive Patch for Allergen Provocation TestDosage form: INJECTABLE, 25 mg edrophonium bromide/2 mlActive substance: edrophoniumManufacturer: Mana Pharma S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.3%/0.3%/0.3% mol/molActive substance: Other diagnostic agentsManufacturer: Sociedad Española De Carburos Metalicos S.A.Prescription requiredDosage form: PULMONARY INHALATION, 0.28%/9.5% mol/molActive substance: Other diagnostic agentsManufacturer: Sociedad Española De Carburos Metalicos S.A.Prescription required
Online doctors for TRUTEST 36 Adhesive Patch for Allergen Provocation Test
Discuss questions about TRUTEST 36 Adhesive Patch for Allergen Provocation Test, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















