TAMIFLU 75 mg HARD CAPSULES

How to use TAMIFLU 75 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Tamiflu 75mg Hard Capsules
oseltamivir
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
?Keep this leaflet, you may need to read it again.
?If you have any further questions, ask your doctor or pharmacist.
?This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
?If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Tamiflu and what is it used for
- What you need to know before you take Tamiflu
- How to take Tamiflu
- Possible side effects
- Storing Tamiflu
- Contents of the pack and other information
1. What is Tamiflu and what is it used for
? Tamiflu is used in adults, adolescents, children, and infants (including full-term newborn babies) to treat flu (influenza). It can be used when you have flu symptoms and it is known that the flu virus is present in the population.
?Tamiflu can also be prescribed to adults, adolescents, children, and infants over 1 year of age to prevent flu on a case-by-case basis, for example, if you have been in contact with someone who has the flu.
?Tamiflu may be prescribed to adults, adolescents, children, and infants (including full-term newborn babies) as preventive treatmentin exceptional circumstances, such as during a global flu epidemic (flu pandemic) and when the seasonal flu vaccine cannot provide sufficient protection.
Tamiflu contains oseltamivir, which belongs to a group of medicines called neuraminidase inhibitors. These medicines prevent the flu virus from spreading in the body. They help to relieve or prevent the symptoms of flu virus infection.
Flu is an infection caused by a virus. The signs of flu often include sudden fever (over 37.8°C), cough, runny or stuffy nose, headache, muscle pain, and extreme tiredness. These symptoms can also be caused by other infections. A true flu infection only occurs during annual outbreaks (epidemics), when flu viruses are widespread in the population. Outside of epidemic periods, flu-like symptoms are generally caused by another type of illness.
2. What you need to know before you take Tamiflu
Do not take Tamiflu:
?if you are allergic(hypersensitive) to oseltamivir or any of the other ingredients of Tamiflu listed in section 6.
If this happens to you, consult your doctor. Do not take Tamiflu.
Warnings and precautions
Before taking Tamiflu, make sure your doctor is informed
?if you are allergic to other medicines
? if you have any kidney disease. If so, your dose may need to be adjusted.
? if you have any severe illness that requires immediate hospitalization.
? if your immune system is not working properly.
? if you have chronic heart or respiratory disease.
During treatment with Tamiflu, tell your doctor immediately
? if you notice changes in your behavior or mood (neuropsychiatric events), especially if they occur in children and adolescents. These can be signs of rare but serious side effects.
Tamiflu is not a flu vaccine
Tamiflu is not a vaccine: it is used to treat the infection or prevent the spread of the flu virus. A vaccine provides you with antibodies against the virus. Tamiflu does not affect the effectiveness of the flu vaccine, and your doctor may prescribe both.
Using Tamiflu with other medicines
Tell your doctor or pharmacist if you are using or have recently used any other medicines, including those obtained without a prescription. This includes:
? chlorpropamide (used to treat diabetes)
? methotrexate (used to treat, for example, rheumatoid arthritis)
? phenylbutazone (used to treat pain and inflammation)
? probenecid (used to treat gout)
Pregnancy and breastfeeding
You should inform your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant, so your doctor can decide if Tamiflu is suitable for you.
The effects on breastfed infants are unknown. You should inform your doctor if you are breastfeeding, so your doctor can decide if Tamiflu is suitable for you.
Consult your doctor or pharmacist before using this medicine.
Driving and using machines
Tamiflu has no effect on your ability to drive or use machines.
Information on some of the ingredients of Tamiflu
This medicine contains less than 1 mmol of sodium (23 mg) per capsule, i.e., it is essentially "sodium-free".
3. How to take Tamiflu
Follow the instructions for taking Tamiflu exactly as your doctor has told you. If you are unsure, consult your doctor or pharmacist.
Take Tamiflu as soon as possible, ideally within 2 days of the onset of flu symptoms.
Recommended doses
For the treatment of flu, take 2 doses a day. It is usually convenient to take one dose in the morning and one in the evening. It is important to complete the entire 5-day treatment, even if you start to feel better quickly.
For patients with weakened immune systems, treatment will continue for 10 days.
For the prevention of flu or after being in contact with an infected person, take 1 dose a day for 10 days. It is best to take this dose in the morning with breakfast.
In special situations, such as widespread flu or patients with weakened immune systems, treatment will continue for up to 6 or 12 weeks.
The recommended dose depends on the patient's body weight.You should use the amount of capsules or oral suspension prescribed by your doctor.
Adults and adolescents 13 years and older
Body weight | Flu treatment: dose for 5 days | Flu treatment (in immunocompromised patients): dose for 10 days | Flu prevention: dose for 10 days |
40 kg or more | 75 mg twice a day | 75 mg twice a day | 75 mg once a day |
*For patients with weakened immune systems, treatment is for 10 days.
75 mg can be obtained with a 30 mg capsule and a 45 mg capsule
Children 1 to 12 years
Body weight | Flu treatment: dose for 5 days | Flu treatment (in immunocompromised patients): dose for 10 days | Flu prevention: dose for 10 days |
10 to 15 kg | 30 mg twice a day | 30 mg twice a day | 30 mg once a day |
Over 15 kg and up to 23 kg | 45 mg twice a day | 45 mg twice a day | 45 mg once a day |
Over 23 kg and up to 40 kg | 60 mg twice a day | 60 mg twice a day | 60 mg once a day |
Over 40 kg | 75 mg twice a day | 75 mg twice a day | 75 mg once a day |
*For children with weakened immune systems, treatment is for 10 days.
**75 mg can be obtained with a 30 mg capsule and a 45 mg capsule
Children under 1 year (0 to 12 months)
Administration of Tamiflu to children under 1 year of age to prevent flu during a pandemic should be done based on the doctor's judgment after considering the potential benefits and risks to the child.
Body weight | Flu treatment: dose for 5 days | Flu treatment (in immunocompromised patients): dose for 10 days | Flu prevention: dose for 10 days |
3 kg to over 10 kg | 3 mg per kg of body weight, twice a day | 3 mg per kg of body weight, twice a day | 3 mg per kg of body weight, once a day |
*For children with weakened immune systems, treatment is for 10 days.
**mg per kg = mg per kilogram of body weight. For example:
If a 6-month-old child weighs 8 kg, the dose is
8 kg x 3 mg per kg = 24 mg
Method of administration
Swallow the capsules whole with water. Do not break or chew the capsules.
Tamiflu can be taken with or without food, although taking it with food may reduce the chance of feeling unwell (nausea or vomiting).
People who may have difficulty swallowing the capsulescan use a liquid medicine, Tamiflu oral suspension, but if this is not available at your pharmacy, you can prepare a liquid form of Tamiflu from the capsules. See the page on Preparing Tamiflu liquid at home.
If you take more Tamiflu than you should
Stop taking Tamiflu and consult a doctor or pharmacist immediately.
In many cases of overdose, no side effects have been reported. When side effects have been reported, they were similar to those that occurred with normal doses and are included in section 4.
Overdose with Tamiflu has been reported more frequently in children than in adults and adolescents. Caution should be exercised when preparing Tamiflu liquid for children and when administering Tamiflu capsules or liquid to children.
If you forget to take Tamiflu
Do not take a double dose to make up for forgotten doses.
If you stop taking Tamiflu
No side effects have been reported when stopping Tamiflu. However, if you stop taking Tamiflu before your doctor told you to, your flu symptoms may return. Always complete the treatment that your doctor has prescribed.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Many of these side effects may be caused by the flu.
Since the marketing of oseltamivir, the following serious side effects have been rarely reported:
? Anaphylactic and anaphylactoid reactions: severe allergic reactions, with swelling of the face and skin, itchy rash, low blood pressure, and difficulty breathing.
? Hepatic disorders (fulminant hepatitis, hepatic function disorder, and jaundice): yellowing of the skin and whites of the eyes, change in stool color, changes in behavior
? Angioedema: severe sudden swelling of the skin, mainly around the head and neck area, including eyes and tongue, with difficulty breathing.
? Stevens-Johnson syndrome and toxic epidermal necrolysis: a complicated allergic reaction that can be life-threatening, severe inflammation of the outer and possibly inner skin, initially with fever, sore throat, and fatigue, skin rash with blistering, peeling, and large areas of skin peeled, possible difficulty breathing and low blood pressure
? Gastrointestinal bleeding: prolonged bleeding from the intestine or vomiting blood
? Neuropsychiatric disorders, as described below.
If you notice any of these symptoms, seek medical help immediately.
The most frequently reported side effects for Tamiflu are feeling unwell or discomfort (nausea, vomiting), stomach pain, stomach upset, headache, and pain. These side effects usually occur after the first dose of the medicine and generally disappear during treatment. The frequency of these side effects is reduced if the medicine is taken with food.
Rare but serious side effects: seek medical help immediately
(These can affect up to 1 in 1,000 people)
During treatment with Tamiflu, rare side effects have been reported, including
? Seizures and delirium, including altered levels of consciousness
?Confusion, abnormal behavior
?Delirium, hallucinations, agitation, anxiety, nightmares
These events were mainly reported in children and adolescents and often started suddenly and resolved quickly. In very rare cases, these events resulted in self-injury, some with a fatal outcome. These neuropsychiatric events have also been reported in patients with flu who were not taking Tamiflu.
? Patients, especially children and adolescents, should be closely monitored for changes in behavior described above.
If you notice any of these symptoms, especially in younger patients, seek medical help immediately.
Adults and adolescents 13 years and older
Very common side effects
(can affect more than 1 in 10 people)
? Headache
? Nausea
Common side effects
(can affect up to 1 in 10 people)
? Bronchitis
? Feverishness
? Cough
? Dizziness
? Fever
? Pain
? Pain in the limbs
? Runny nose
? Difficulty sleeping
? Sore throat
? Stomach pain
? Fatigue
? Feeling full in the upper abdomen
? Infections of the upper respiratory tract (inflammation of the nose, throat, and sinuses)
? Stomach upset
? Vomiting
Uncommon side effects
(can affect up to 1 in 100 people)
? Allergic reactions
? Altered level of consciousness
? Seizure
? Heart rhythm disorders
? Liver function disorders, from mild to severe
? Skin reactions (skin inflammation, red itchy rash, scaly skin)
Rare side effects
(can affect up to 1 in 1,000 people)
? Thrombocytopenia (low platelet count)
? Vision disorders
Children 1 to 12 years
Very common side effects
(can affect more than 1 in 10 people)
? Cough
? Runny nose
? Vomiting
Common side effects
(can affect up to 1 in 10 people)
? Conjunctivitis (red eyes and tearing or eye pain)
? Ear inflammation and other ear disorders
? Headache
? Nausea
? Runny nose
? Stomach pain
? Feeling full in the upper abdomen
? Stomach upset
Uncommon side effects
(can affect up to 1 in 100 people)
? Skin inflammation
? Eardrum disorder (eardrum)
Infants under 1 year
The side effects reported in children from 0 to 12 months of age are mostly similar to those reported in older children (from 1 year onwards). Additionally, diarrhea and diaper dermatitis have been reported.
If you think any of the side effects you are experiencing are serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
However,
?if you or your child are sick several times, or
?if flu symptoms worsen or fever continues
Tell your doctor as soon as possible.
Reporting side effects
If you experience any side effects, talk to your doctor, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Tamiflu
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of the month stated.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Tamiflu Composition
Each hard capsule contains oseltamivir equivalent to 75 mg of oseltamivir.
The other components are:
capsule content: pregelatinized starch, talc, povidone, sodium croscarmellose, and sodium stearyl fumarate
capsule shell: gelatin, yellow iron oxide (E172), red iron oxide (E172), black iron oxide (E172), and titanium dioxide (E171)
printing ink: shellac (E904), titanium dioxide (E171), and FD & C Blue 2 (carmine indigo, E132).
Product Appearance and Container Contents
The 75 mg hard capsules are formed by a gray opaque body with the inscription “ROCHE” and a light yellow opaque cap with the inscription “75 mg”. The inscriptions are blue.
Tamiflu 75 mg hard capsules are available in blisters of 10 capsules.
Marketing Authorization Holder and Manufacturer
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Roche Pharma AG
Emil-Barell-Str. 1,
D-79639 Grenzach-Wyhlen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien N.V. Roche S.A. Tel: +32 (0) 2 525 82 11 | Lithuania UAB “Roche Lietuva” Tel: +370 5 2546799 |
Bulgaria Roche Bulgaria EOOD Tel: +359 2 818 44 44 | Luxembourg/Luxemburg (See Belgium/Belgien) |
Czech Republic Roche s. r. o. Tel: +420 - 2 20382111 | Hungary Roche (Hungary) Kft. Tel: +36 - 1 279 4500 |
Denmark Roche Pharmaceuticals A/S Tel: +45 - 36 39 99 99 | Malta (See Ireland) |
Germany Roche Pharma AG Tel: +49 (0) 7624 140 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Estonia Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norway Roche Norge AS Tel: +47 - 22 78 90 00 |
Greece Roche (Hellas) A.E. Tel: +30 210 61 66 100 | Austria Roche Austria GmbH Tel: +43 (0) 1 27739 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Poland Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tel: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Croatia Roche d.o.o Tel: +385 1 4722 333 | Romania Roche România S.R.L. Tel: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenia Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Tel: +354 540 8000 | Slovakia Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italy Roche S.p.A. Tel: +39 - 039 2471 | Finland Roche Oy Tel: +358 (0) 10 554 500 |
Cyprus Γ.Α.Σταμ?της & Σια Λτδ. Tel: +357 - 22 76 62 76 | Sweden Roche AB Tel: +46 (0) 8 726 1200 |
Latvia Roche Latvija SIA Tel: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of Last Revision of this Leaflet:
Detailed information on this drug is available on the European Medicines Agency website: http://www.ema.europa.eu/.
--------------------------------------------------------------------------------------------------------------------------
Information for the User
For people who find it difficult to swallow the capsules,including very young children, there is a liquid medicine, Tamiflu oral suspension.
If you need a liquid medicine, but it is not available, a suspension can be prepared in the pharmacy from Tamiflu capsules (see Information for Healthcare Professionals). The pharmacy-prepared preparation is the recommended option.
If the pharmacy preparation is also not available, you can prepare Tamiflu liquid at home from these capsules.
The dose is the same for treatment as for prevention of the flu. The difference is the frequency of administration.
Preparing Tamiflu Liquid at Home
? If you have the correct capsulefor the required dose (a 75 mg dose), open the capsule and mix its contents in a teaspoon (or less) of suitable sweetened food.
This is usually suitable for children over 1 year old. See the top part of the instructions.
? If you need smaller doses, preparing Tamiflu liquid from the capsules requires more steps.
This is suitable for small children and babies: they need a Tamiflu dose of less than 30 mg. See the bottom part of the instructions.
Adults, Adolescents 13 Years and Older, and Children Weighing 40 kg or More
To Prepare a 75 mg Dose, You Will Need:
?One 75 mg Tamiflu Capsule
?Sharp Scissors
?A Small Container
?A 5 ml Spoon
?Water
?Sweetened Foodto mask the bitter taste of the powder
Examples: chocolate syrup or cherry syrup and dessert sauce, such as caramel or condensed milk.
Or you can prepare sugared water: mix one spoonful of water with three-quarters (3/4) of a spoonful of sugar.
Step 1: Check the Correct Dose
To know the correct amount to use, look for the patient's weight on the left side of the table.
Look at the right column to check the number of capsules to give the patient for a single dose. The dose is the same for treatment as for prevention of the flu.
75 mg dose |
|
You should only use the 75 mg capsules for 75 mg doses. Do not attempt to prepare 75 mg doses using the contents of the 30 mg or 45 mg capsules.
Weight | Tamiflu Dose | Number of Capsules |
40 kg or more | 75 mg | 1 capsule |
Not for Children Weighing Less than 40 kg
If you need to prepare a dose less than 75 mg for children weighing less than 40 kg. See below
Step 2: Pour All the Powder into the Container
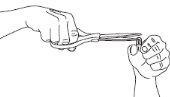
Hold a 75 mg Tamiflu Capsulevertically over a container and carefully cut off the rounded end with scissors.
Pour all the powder into the container.
Be careful with the powder, as it can be irritating to the skin and eyes.
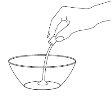
Step 3: Sweeten the Powder and Give the Dose
Add a small amount – no more than one spoonful – of sweetened food to the powder in the container.
This is to mask the bitter taste of the Tamiflu powder.
Mix well.
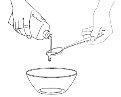
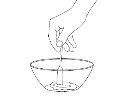
Give the Patient the Entire Contentsof the container immediately.
If There is Any Mixture Leftin the container, rinse the container with a small amount of water and give it to the patient to take all of it. Repeat this procedure each time you need to give the medication.
Infants Less than 1 Year Old and Children Weighing Less than 40 kg
To Prepare a Smaller Single Dose,
You Will Need:
?A 75 mg Tamiflu Capsule
?Sharp Scissors
?Two Small Containers
?A Large Oral Dosing Dispenser to Measure Water - a 5 or 10 ml Dispenser
?A Small Oral Dispenser that Shows 0.1 ml Measurements, to Give the Dose
?A 5 ml Spoon
?Water
?Sweetened Foodto mask the bitter taste of Tamiflu
Examples: chocolate syrup or cherry syrup and dessert sauce, such as caramel or condensed milk.
Or you can prepare sugared water by mixing one spoonful of water with three-quarters (3/4) of a spoonful of sugar.
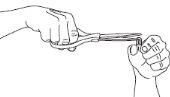
Step 1: Pour All the Powder into a Container
Hold a 75 mg capsule vertically over one of the containers and carefully cut off the rounded end with scissors. Be careful with the powder, as it can be irritating to the skin and eyes.
Pour all the powder into the container, regardless of the dose you are preparing.
The amount is the same for treatment as for prevention of the flu.
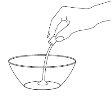
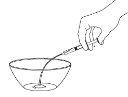
Step 2: Add Water to Dilute the Medication
Use the larger dispenser to draw 12.5 mlof water. Add the water to the powder in the container. |
|
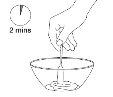
Shake the suspension with a spoon for 2 minutes. |
|
Do not worry if not all the powder dissolves. The undissolved powder corresponds to inactive ingredients.
Step 3: Choose the Correct Dose for the Child's Weight
Look for the child's weight in the left column of the table.
The right column of the table shows how much liquid mixture you need to prepare.
Infants Less than 1 Year Old (Including Full-Term Newborns)
Child's Weight (Closest) | Amount of Mixture to Prepare |
3 kg | 1.5 ml |
3.5 kg | 1.8 ml |
4 kg | 2.0 ml |
4.5 kg | 2.3 ml |
5 kg | 2.5 ml |
5.5 kg | 2.8 ml |
6 kg | 3.0 ml |
6.5 kg | 3.3 ml |
7 kg | 3.5 ml |
7.5 kg | 3.8 ml |
8 kg | 4.0 ml |
8.5 kg | 4.3 ml |
9 kg | 4.5 ml |
9.5 kg | 4.8 ml |
10 kg or more | 5.0 ml |
Children 1 Year or Older Who Weigh Less than 40 kg
Child's Weight (Closest) | Amount of Mixture to Prepare |
Up to 15 kg | 5.0 ml |
15 to 23 kg | 7.5 ml |
23 to 40 kg | 10.0 ml |
Step 4: Prepare the Liquid Mixture
Make sure you have the correct dispenser size.
Prepare the correct amount of liquid mixture from the first container.
Prepare it carefully to avoid getting air bubbles.
Gently add the correct dose to the second container.
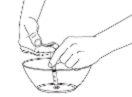
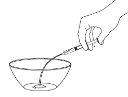
Step 5: Sweeten and Give to the Child
Add a small amount – no more than one spoonful – of sweetened food to the second container.
This is to mask the bitter taste of the Tamiflu suspension.
Mix well.
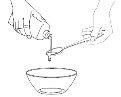
Give the Child the Entire Contentsof the second container (sweetened food with Tamiflu liquid) immediately.
If There is Any Mixture Leftin the second container, rinse the container with a small amount of water and have the child drink all of it. For children who cannot drink directly from the container, give it to them with a spoon or use an appropriate bottle to give the child the leftover liquid.
Give the child something to drink.
Discard any Remaining Liquidfrom the first container.
Repeat this procedure each time you need to give the medication.
--------------------------------------------------------------------------------------------------------------
Information for Healthcare Professionals Only
Patients Who Cannot Swallow Capsules:
The commercially available Tamiflu oral suspension (6 mg/ml) is the preferred medication for pediatric and adult patients who have difficulty swallowing capsules or require lower doses. If Tamiflu oral suspension is not available, the pharmacist can prepare a suspension (6 mg/ml) from Tamiflu capsules. If the pharmacy-prepared suspension is also not available, patients can prepare the suspension at home from the capsules.
Oral Dosing Dispensers(oral syringes) with the correct volume and graduation should be provided to administer the pharmacy-prepared suspension and for the procedures to follow for home preparation. In both cases, the volumes needed should preferably be marked on the dispensers. For home preparation, separate dispensers should be provided to take the correct volume of water and to measure the Tamiflu mixture with water. For measuring 12.5 ml of water, 10 ml dispensers should be used.
The following is the suitable dispenser size to draw the correct volume of Tamiflu suspension (6 mg/ml).
Infants Less than 1 Year Old (Including Full-Term Newborns):
Tamiflu Dose | Amount of Tamiflu Suspension | Dispenser Size to Use (0.1 ml Graduation) |
9 mg | 1.5 ml | 2.0 ml (or 3.0 ml) |
10 mg | 1.7 ml | 2.0 ml (or 3.0 ml) |
11.25 mg | 1.9 ml | 2.0 ml (or 3.0 ml) |
12.5 mg | 2.1 ml | 3.0 ml |
13.75 mg | 2.3 ml | 3.0 ml |
15 mg | 2.5 ml | 3.0 ml |
16.25 mg | 2.7 ml | 3.0 ml |
18 mg | 3.0 ml | 3.0 ml (or 5.0 ml) |
19.5 mg | 3.3 ml | 5.0 ml |
21 mg | 3.5 ml | 5.0 ml |
22.5 mg | 3.8 ml | 5.0 ml |
24 mg | 4.0 ml | 5.0 ml |
25.5 mg | 4.3 ml | 5.0 ml |
27 mg | 4.5 ml | 5.0 ml |
28.5 mg | 4.8 ml | 5.0 ml |
30 mg | 5.0 ml | 5.0 ml |
Children 1 Year or Older Who Weigh Less than 40 kg:
Tamiflu Dose | Amount of Tamiflu Suspension | Dispenser Size to Use (0.1 ml Graduation) |
30 mg | 5.0 ml | 5.0 ml |
5.0 ml (or 10.0 ml) | 45 mg | 7.5 ml | 10.0 ml |
60 mg | 10.0 ml | 10.0 ml |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TAMIFLU 75 mg HARD CAPSULESDosage form: CAPSULE, 30 mgActive substance: oseltamivirManufacturer: Actavis Group Ptc Ehf.Prescription requiredDosage form: CAPSULE, 75 mgActive substance: oseltamivirManufacturer: Actavis Group Ptc Ehf.Prescription requiredDosage form: CAPSULE, 30 mgActive substance: oseltamivirManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for TAMIFLU 75 mg HARD CAPSULES
Discuss questions about TAMIFLU 75 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions