TAKHZYRO 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use TAKHZYRO 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
TAKHZYRO300mg solution for injection in pre-filled syringe
lanadelumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is TAKHZYRO and what is it used for
- What you need to know before you use TAKHZYRO
- How to use TAKHZYRO
- Possible side effects
- Storage of TAKHZYRO
- Contents of the pack and further information
- Instructions for use
1. What is TAKHZYRO and what is it used for
TAKHZYRO contains the active substance lanadelumab.
What is TAKHZYRO used for
TAKHZYRO is a medicine used in patients from 2 years of age with hereditary angioedema (HAE) to prevent attacks of angioedema.
What is hereditary angioedema (HAE)
HAE is a hereditary disease within a family. When you have this disease, there is not enough of a protein called "C1 inhibitor" in the blood or the C1 inhibitor does not work properly. This leads to an excess of "plasma kallikrein", which in turn produces higher levels of "bradykinin" in the bloodstream. Too much bradykinin causes HAE symptoms, such as swelling and pain in:
- the hands and feet
- the face, eyelids, lips, or tongue
- the vocal cords (larynx), which can make it difficult to breathe
- the genitals
How TAKHZYRO works
TAKHZYRO is a type of protein that blocks the activity of plasma kallikrein, which helps to reduce the amount of bradykinin in the bloodstream and prevents HAE symptoms.
2. What you need to know before you use TAKHZYRO
Do not use TAKHZYRO
If you are allergic to lanadelumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
- Talk to your doctor, pharmacist, or nurse before starting to use TAKHZYRO.
- If you have a severe allergic reaction to TAKHZYRO with symptoms such as rash, chest tightness, wheezing, or rapid heartbeat, tell your doctor, pharmacist, or nurse immediately.
Keep a record
It is strongly recommended that each time you are given a dose of TAKHZYRO, you note the name and batch number of the medicine, so that you have a record of the batches used.
Lab tests
Tell your doctor if you are using TAKHZYRO before undergoing lab tests to evaluate your blood clotting, as the presence of TAKHZYRO in the blood may interfere with certain lab tests and lead to inaccurate results.
Children and adolescents
TAKHZYRO is not recommended for use in children under 2 years of age, as it has not been studied in this age group.
Other medicines and TAKHZYRO
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
It is not known whether TAKHZYRO affects other medicines or is affected by other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. The information on the safety of using TAKHZYRO during pregnancy and breastfeeding is limited. As a precautionary measure, it is preferable to avoid the use of lanadelumab during pregnancy and breastfeeding. Your doctor will discuss with you the risks and benefits of receiving this medicine.
Driving and using machines
The influence of this medicine on the ability to drive and use machines is negligible.
TAKHZYRO contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per pre-filled syringe; i.e., it is essentially "sodium-free".
3. How to use TAKHZYRO
TAKHZYRO is available as a pre-filled syringe as a ready-to-use solution. A doctor experienced in the care of patients with HAE will initiate and supervise your treatment.
Follow the instructions for administration of the medicine contained in this leaflet or as told by your doctor, pharmacist, or nurse. If you have any doubts or other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
How much TAKHZYRO to use
For adults and adolescents from 12 years to less than 18 years of age:
- The recommended starting dose is 300 mg of lanadelumab every 2 weeks. If you have not had any attacks for a long period, your doctor may adjust the dose to 300 mg of lanadelumab every 4 weeks, especially if your body weight is low.
- In patients with a body weight below 40 kg, a starting dose of 150 mg of lanadelumab every 2 weeks may also be considered. If you have not had any attacks for a long period, your doctor may adjust the dose to 150 mg of lanadelumab every 4 weeks.
For children from 2 years to less than 12 years of age, the recommended starting dose is based on body weight:
Body weight (kg) | Recommended starting dose | Dose adjustment |
10 to less than 20 kg | 150 mg of lanadelumab every 4 weeks | A dose increase to 150 mg of lanadelumab every 3 weeks may be considered in patients with insufficient control of attacks |
20 to less than 40 kg | 150 mg of lanadelumab every 2 weeks | A dose reduction to 150 mg of lanadelumab every 4 weeks may be considered in stable patients without attacks under treatment |
40 kg or more | 300 mg of lanadelumab every 2 weeks | A dose reduction to 300 mg of lanadelumab every 4 weeks may be considered in stable patients without attacks under treatment |
- In patients with a body weight of 20 to less than 40 kg who have not had any attacks for a long period, the doctor may allow your child to continue receiving the same dose when they reach 12 years of age.
How to inject TAKHZYRO
If you self-inject TAKHZYRO or if it is injected by your caregiver, you or your caregiver must read and follow the instructions in section 7, "Instructions for use" carefully.
- TAKHZYRO is injected under the skin ("subcutaneous injection").
- The injection can be given by you or a caregiver in patients from 12 years of age.
- The injection can be given by a healthcare professional or by a caregiver in patients from 2 years to less than 12 years of age.
- A doctor, pharmacist, or nurse should teach you how to prepare and inject TAKHZYRO correctly before you use it for the first time. Do not inject yourself or inject another person until you have been taught how to inject the medicine.
- Insert the needle into the fatty tissue of the stomach (abdomen), thigh, or upper arm.
- Inject the medicine into a different place each time.
- Use each TAKHZYRO pre-filled syringe only once.
If you use more TAKHZYRO than you should
Tell your doctor, pharmacist, or nurse if you have been given a dose of TAKHZYRO that is higher than recommended.
If you forget to use TAKHZYRO
If you miss a dose of TAKHZYRO, inject the dose as soon as possible. The administration of the next doses may require an adjustment according to the desired dosing frequency, making sure to:
- wait at least 10 days between doses in patients with a dosing regimen every 2 weeks,
- wait at least 17 days between doses in patients with a dosing regimen every 3 weeks,
- wait at least 24 days between doses in patients with a dosing regimen every 4 weeks.
If you are not sure when to inject TAKHZYRO after missing a dose, ask your doctor, pharmacist, or nurse.
If you stop using TAKHZYRO
It is important that you keep injecting TAKHZYRO as your doctor has told you, even if you feel better. If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you have a severe allergic reaction to TAKHZYRO with symptoms such as rash, chest tightness, wheezing, or rapid heartbeat, tell your doctor, pharmacist, or nurse immediately.
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects.
Very common (may affect more than 1 in 10 people):
- Reactions at the injection site: symptoms are pain, redness of the skin, bruising, discomfort, swelling, bleeding, itching, hardening of the skin, tingling, warmth, and rash.
Common (may affect up to 1 in 10 people):
- Allergic reactions, such as itching, discomfort, and tingling in the tongue
- Dizziness, feeling of fainting
- Raised rash
- Muscle pain
- Lab test results showing changes in the liver
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of TAKHZYRO
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month shown.
TAKHZYRO 300 mg solution for injection in pre-filled syringe
Store in a refrigerator (2°C to 8°C). Do not freeze. Keep the pre-filled syringe in the outer packaging to protect it from light.
The pre-filled syringes can be stored below 25°C for a single period of 14 days, but not beyond the expiry date.
After storage at room temperature, do not refrigerate TAKHZYRO again for storage.
When a pre-filled syringe is removed from the refrigerator from a multiple pack, the remaining pre-filled syringes should be returned to the refrigerator for when they are to be used in the future.
Do not use this medicine if you notice signs of deterioration, such as particles in the pre-filled syringe or a change in the color of the injection solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of TAKHZYRO
- The active ingredient is lanadelumab. Each prefilled syringe contains 300 mg of lanadelumab in a 2 ml solution.
- The other components are disodium phosphate dihydrate, citric acid monohydrate, histidine, sodium chloride, polysorbate 80, and water for injectable preparations; see section 2 "TAKHZYRO contains sodium"
Appearance of the Product and Container Contents
TAKHZYRO is presented as a clear, colorless to pale yellow injectable solution in a prefilled syringe.
TAKHZYRO is available as:
- a single-unit package containing one 2 ml prefilled syringe in a box
- a single-unit package containing two 2 ml prefilled syringes in a box
- multiple-unit packages containing 3 intermediate cartons, each with two 2 ml prefilled syringes.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Takeda Pharmaceuticals International AG Ireland Branch
Block 2 Miesian Plaza
50-58 Baggot Street Lower
Dublin 2
D02 HW68
Ireland
Manufacturer
Takeda Pharmaceuticals International AG Ireland Branch
Block 2 Miesian Plaza
50-58 Baggot Street Lower
Dublin 2
D02 HW68
Ireland
Shire Pharmaceuticals Ireland Limited
Blocks 2 & 3 Miesian Plaza
50-58 Baggot Street Lower
Dublin 2
Ireland
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Takeda Belgium NV Tel: +32 2 464 06 11 | Lietuva Takeda, UAB Tel: +370 521 09 070 [email protected] |
| Luxembourg/Luxemburg Takeda Belgium NV Tel: +32 2 464 06 11 [email protected] |
Ceská republika Takeda Pharmaceuticals Czech Republic s.r.o. Tel: + 420 234 722 722 [email protected] | Magyarország Takeda Pharma Kft. Tel.: +36 1 270 7030 [email protected] |
Danmark Takeda Pharma A/S Tlf: +45 46 77 10 10 [email protected] | Malta Drugsales Ltd Tel: +356 21419070 |
Deutschland Takeda GmbH Tel: +49 (0)800 825 3325 [email protected] | Nederland Takeda Nederland B.V. Tel: +31 20 203 5492 [email protected] |
Eesti Takeda Pharma OÜ Tel: +372 6177 669 [email protected] | Norge Takeda AS Tlf.: +47 800 800 30 [email protected] |
Ελλάδα Τakeda ΕΛΛΑΣ Α.Ε. Τηλ: +30 210 6387800 [email protected] | Österreich Takeda Pharma Ges.m.b.H. Tel: +43 (0) 800-20 80 50 [email protected] |
España Takeda Farmacéutica España, S.A. Tel: +34 917 90 42 22 [email protected] | Polska Takeda Pharma Sp. z o.o. Tel.: +48223062447 [email protected] |
France Takeda France SAS Tél: + 33 1 40 67 33 00 [email protected] | Portugal Takeda Farmacêuticos Portugal, Lda. Tel: + 351 21 120 1457 [email protected] |
Hrvatska Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 [email protected] | România Takeda Pharmaceuticals SRL Tel: +40 21 335 03 91 |
Ireland Takeda Products Ireland Ltd Tel: 1800 937 970 [email protected] | Slovenija Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel: + 386 (0) 59 082 480 [email protected] |
Ísland Vistor ehf. Sími: +354 535 7000 [email protected] | Slovenská republika Takeda Pharmaceuticals Slovakia s.r.o. Tel: +421 (2) 20 602 600 [email protected] |
Italia Takeda Italia S.p.A. Tel: +39 06 502601 [email protected] | Suomi/Finland Takeda Oy Puh/Tel: 0800 774 051 [email protected] |
Κύπρος Proton Medical (Cyprus) Ltd Τηλ: +357 22866000 | Sverige Takeda Pharma AB Tel: 020 795 079 |
Latvija Takeda Latvia SIA Tel: +371 67840082 [email protected] | United Kingdom (Northern Ireland) Takeda UK Ltd Tel: +44 (0) 3333 000 181 [email protected] |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Instructions for Use
Make sure to read, understand, and follow the instructions for use before injecting TAKHZYRO. Contact your healthcare professional if you have questions.
Intended Use
The TAKHZYRO prefilled syringe is a single-dose injection device with a needle, ready for use, intended for subcutaneous administration of the medicinal product by healthcare professionals and caregivers or for self-administration (for patients 12 years of age and older).
Storage of TAKHZYRO
- Store the TAKHZYRO prefilled syringe in the refrigerator at 2 °C to 8 °C. Do notfreeze.
- A prefilled syringe that has been removed from the refrigerator must be stored at less than 25 °C and used within 14 days. After storage at room temperature, do not refrigerate TAKHZYRO again for storage.
- When a prefilled syringe is removed from the refrigerator from a multiple-unit package, the remaining prefilled syringes should be returned to the refrigerator for future use.
- Store TAKHZYRO in the original carton to protect the prefilled syringe from light.
- Discard the TAKHZYRO prefilled syringe if it has not been refrigerated, has been frozen, or has not been stored in its original carton protected from light.
- Do notshake TAKHZYRO.
- Keep TAKHZYRO and all medicines out of the reach of children.
Parts of Your TAKHZYRO Prefilled Syringe Before Use (Figure A).
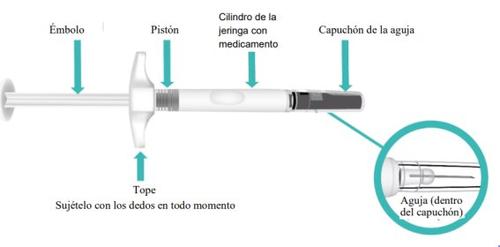
Figure A: TAKHZYRO Prefilled Syringe
STEP 1: Preparation of the Injection
- Take an alcohol swab, a cotton pad or ball, an adhesive bandage, and a sharps disposal container (Figure B) and place them on a flat, clean surface in a well-lit area. These items are not included in the TAKHZYRO packaging.

Figure B: Supplies
- Remove the TAKHZYRO prefilled syringe from the refrigerator.
- Do notuse the TAKHZYRO prefilled syringe if the security seal is open or broken.
- Before preparing the injection, wait for the prefilled syringe to reach room temperature for at least 15 minutes.
- This medicinal product is heat-sensitive. Do notuse heat sources, such as a microwave or hot water, to warm the TAKHZYRO prefilled syringe.
- Do notremove the needle shield until you are ready to start the injection.

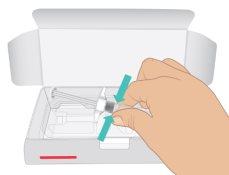
- Open the carton. Hold the syringe barrel and pull out the TAKHZYRO prefilled syringe from the tray (Figure C).
|
Figure C: Remove the Prefilled Syringe |
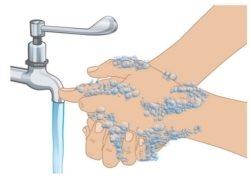
- Wash your hands with soap and water (Figure D). Dry your hands completely.
- Do nottouch anysurface or part of your body after washing your hands before starting the injection.
|
Figure D: Hand Washing |
- Check the expiration date(EXP) on the syringe barrel (Figure E).
Do notuse the TAKHZYRO prefilled syringe if the expiration date has passed. If the TAKHZYRO prefilled syringe is expired, discard it in a sharps disposal container and contact your healthcare professional.
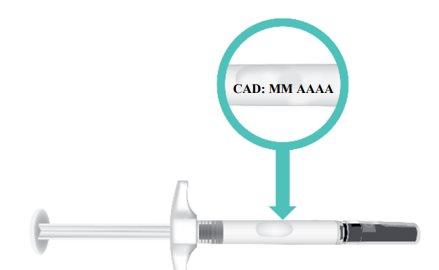
Figure E: Location of Expiration Date
- Inspectthe TAKHZYRO prefilled syringe for damage and ensure the medicinal product is colorless or pale yellow (Figure F).
- Do notuse the product if the syringe is damaged, for example, if it is cracked.
- Do notuse the TAKHZYRO prefilled syringe if the medicinal product has changed color, is cloudy, or contains particles or residue.
- You may see air bubbles in your TAKHZYRO prefilled syringe. This is normal and will not affect the dose.
If you cannot use the prefilled syringe, contact your healthcare professional.
|
Figure F: Inspect the Prefilled Syringe |
STEP 2: Selection and Preparation of the Injection Site
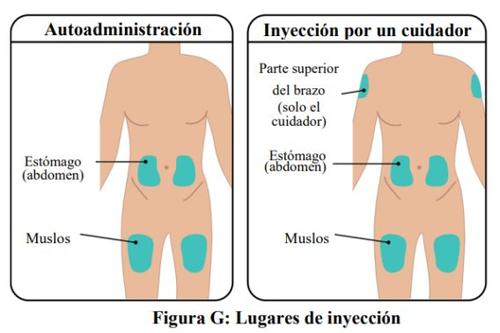
- The TAKHZYRO prefilled syringe should only be injected into the following sites (Figure G):
- stomach (abdomen)
- thigh
- upper arm (only if the injection is administered by a healthcare professional or caregiver)
- Do notapply the injection to an area of the body where the skin is irritated, red, infected, or bruised.
- The injection site you choose should be at least 5 cm away from any scar or the navel.
Important:
Rotate the injection sites to keep the skin healthy. Each new injection should be administered at least 3 cm away from the last site used.
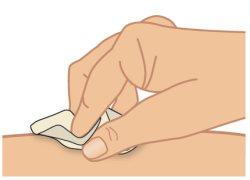
- Clean the injection site with an alcohol swab and wait for it to dry completely (Figure H).
- Do notblow or fan the cleaned area.
- Do nottouch this area again before administering the injection.
|
Figure H: Clean the Injection Site |
- Carefully pull off the needle shield with one hand while firmly holding the middle part of the TAKHZYRO prefilled syringe with the other. Discard the needle shield in the trash or a sharps disposal container (Figure I).
- Do nottouch or press the plunger until you are ready to start the injection.
- To avoid injuring yourself with the needle, do notrecap the TAKHZYRO prefilled syringe.
- Do notuse the TAKHZYRO prefilled syringe if it has been dropped without the needle shield.
- Do notuse the TAKHZYRO prefilled syringe if the needle appears damaged or bent.
- Do nottouch the needle or let the needle come into contact with anything.
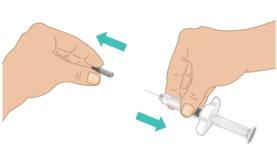
Figure I: Remove the Needle Shield
STEP 3: Injection of TAKHZYRO
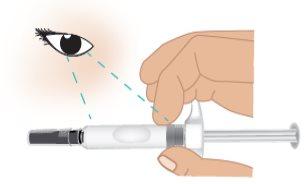
- Hold the TAKHZYRO prefilled syringe with one hand like a pencil (Figure J). Avoid touching the needle or pushing the plunger.
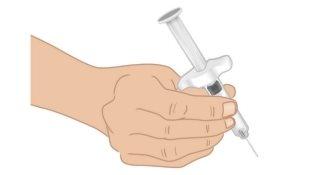
Figure J: Hold the Prefilled Syringe
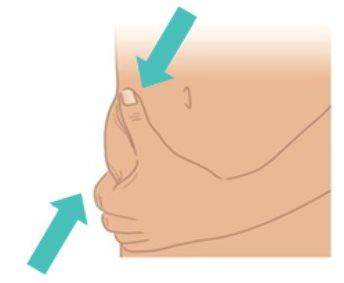
- Use your other hand to gently pinch the skin, creating a fold of about 3 cm at the injection site you cleaned.
- Maintain the fold until the injection is complete and the needle is removed (Figure K).
|
Figure K: Pinch a 3 cm Skin Fold |
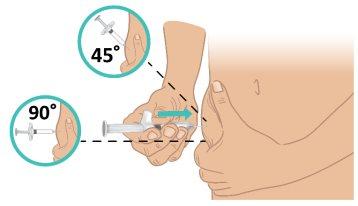
- With a quick, firm motion, insert the needle into the skin at a 45- to 90-degree angle. Make sure to keep the needle in place (Figure L).
Important:Inject directly into the fatty tissue under the skin (subcutaneous injection). |
|
Figure L: Insert the Needle |
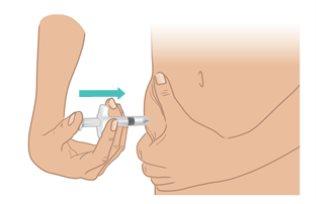
- Slowly pushthe plunger until it stops(Figure M).
- Slowly pull out the needle while keeping the syringe at the same angle. Gently release the skin fold.
Important:Do notremove the needle until all the liquid has been injected and the syringe is empty. |
| |
Figure M: Push the Plunger until it Stops |
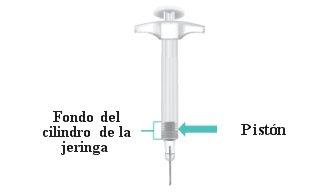
When the injection is complete, the piston will be at the bottom of the syringe barrel (Figure N). |
|
Figure N: Piston at the Bottom of the Syringe Barrel |
Important: Always keep the sharps disposal container out of the reach of children. |
|
Figure O: Dispose of in a Sharps Disposal Container |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TAKHZYRO 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 150 mgActive substance: lanadelumabManufacturer: Takeda Pharmaceuticals International Ag Ireland BranchPrescription requiredDosage form: INJECTABLE, 300 mgActive substance: lanadelumabManufacturer: Takeda Pharmaceuticals International Ag Ireland BranchPrescription requiredDosage form: INJECTABLE, 200 mgActive substance: Drugs used in hereditary angioedemaManufacturer: Csl Behring GmbhPrescription required
Online doctors for TAKHZYRO 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about TAKHZYRO 300 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions