
SYNTHAMIN 17 REFORMULATED WITHOUT ELECTROLYTES SOLUTION FOR INFUSION

How to use SYNTHAMIN 17 REFORMULATED WITHOUT ELECTROLYTES SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SYNTHAMIN 17 REFORMULATED WITHOUT ELECTROLYTES solution for infusion
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet:
- What is Synthamin and what is it used for
- What you need to know before you are given Synthamin
- How Synthamin will be given to you
- Possible side effects
- Storage of Synthamin
- Contents of the pack and other information
1. What is Synthamin and what is it used for
Synthamin is a sterile solution that contains a large number of compounds called amino acids. These are the building blocks for proteins that are vital for your body. It also contains some chemical substances called electrolytes, which are very important for your body to function properly.
Together with other compounds such as minerals and vitamins, it is used to provide nutrition (food) directly into your blood when you cannot take food by mouth. It is important that you are given this medicine to help you improve.
It is usually given together with other nutrition solutions.
2. What you need to know before you are given Synthamin
Synthamin should not be given to you if you have any of the following conditions
- If you are allergic to the active substances or to any of the other components of this medicine (listed in section 6).
medicine (including those listed in section 6).
- If you have any disease in amino acid metabolism.
Warnings and precautions
Talk to your doctor or nurse before you start using Synthamin
- Synthamin will only be used if the solution is clear and the packaging is not damaged.
- If symptoms of pulmonary disorders appear.
- If you have a fever or feel unwell.
- You will have regular blood and urine tests to ensure you are receiving the correct amount of the solution. If necessary, you will be given other treatments.
- You will be closely monitored if you have liver, heart, or kidney problems. Please inform your doctor about this.
- You will be carefully monitored if you have diabetes.
- If it is being given to a very small child, more checks will be carried out.
- If necessary, you may also be given a vitamin called folic acid, fatty acids (the building blocks of fats), and sugar solutions (such as glucose) to ensure your body has all the necessary elements for good health.
- Synthamin should not be given at the same time as, before, or after a blood transfusion through the same infusion equipment.
- The combined administration of Synthamin injectable solutions with concentrated glucose solutions may lead to hyperglycemia, glucosuria, and hyperosmolar syndrome. Therefore, in patients receiving this treatment, routine blood and urine glucose monitoring should be performed. In certain patients, exogenous insulin administration may be necessary.
If any abnormal signs or symptoms of hypersensitivity or allergic reactions are observed, the infusion should be stopped immediately.
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) should be protected from light exposure until the end of administration (see section 2).
Using Synthamin with other medicines
Tell your doctor or nurse if you are taking or have recently taken or might take any other medicines.
No interaction studies have been performed with Synthamin.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
The safety of using amino acid solutions in pregnant or breastfeeding women has not been established. Your doctor will carefully weigh the benefits and potential risks before prescribing Synthamin.
Driving and using machines
Synthamin does not affect your ability to drive or use machines.
3. How Synthamin will be given to you
Use in adults
Your doctor will determine an infusion rate based on your needs and clinical condition, which will depend on your weight, your body's needs, the amount of sugar solution (such as glucose) you can be given, and the reason for your treatment.
The solution will be given to you through a slow drip injection (called infusion) into a large vein in your chest (the superior vena cava). The drip rate will not be more than 70 ml per hour, and you will not receive more than 40 ml per kilogram of body weight in a day.
You will not receive blood transfusions through this tube.
Use in children
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) should be protected from light exposure until the end of administration (see section 2).
In children, the dose of parenteral nutrition should be adjusted individually based on the patient's requirements for amino acids, electrolytes, and energy.
If you are given too much Synthamin
Since your doctor will be the one giving you Synthamin, it is unlikely that you will be given too much. However, if you think you have received more than you should, tell your doctor or nurse.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Telephone 915.620.420.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
There have been reports of a stinging pain and redness at the site where the medicine is injected into your vein. Hypersensitivity reactions, including a severe allergic reaction called anaphylaxis, and other skin reactions (urticaria, rash, pruritus, erythema), gastrointestinal reactions, and circulatory (shock) or respiratory manifestations have also been reported.
You may experience:
- fever,
- chills,
- high blood pressure (hypertension) or low blood pressure (hypotension),
- joint pain (arthralgia),
- muscle pain (myalgia),
- headache (cephalalgia),
- increased levels of nitrogen in the blood (azotemia) or ammonia (hyperammonemia),
- liver disorders.
- venous irritation (thrombosis, pain, erythema, heat, inflammation, hardening).
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report them directly to the Spanish Medicines Agency's website www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Synthamin
Keep this medicine out of the sight and reach of children.
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) should be protected from light exposure until the end of administration (see section 2).
Do not store above 25°C.
Protect from light until immediately before use.
Do not refrigerate.
Do not use this medicine after the expiry date which is stated on the bag and carton after EXP. The expiry date is the last day of the month shown.
Discard partially used containers. Any remaining solution should be disposed of by a healthcare professional.
6. Contents of the pack and other information
Composition of Synthamin
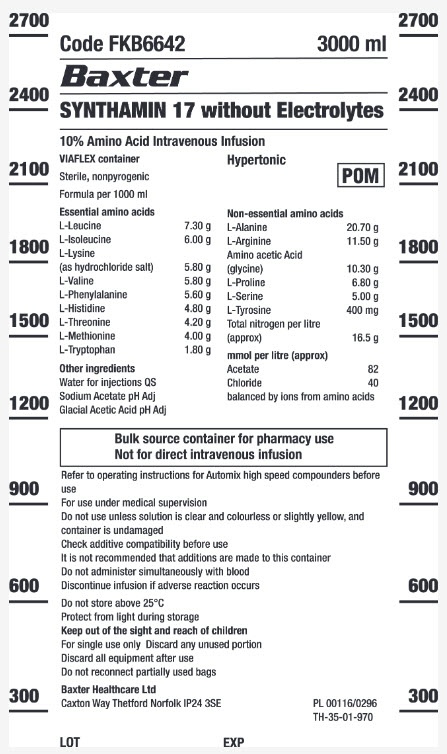
The active substances per 1000 ml are: Glycine 10.30 g, L-Alanine 20.70 g, L-Arginine 11.50 g, L-Phenylalanine 5.60 g, L-Histidine 4.80 g, L-Isoleucine 6.00 g, L-Leucine 7.30 g, L-Lysine hydrochloride 7.26 g, L-Methionine 4.00 g, L-Proline 6.80 g, L-Serine 5.00 g, L-Tyrosine 400 mg, L-Threonine 4.20 g, L-Tryptophan 1.80 g, L-Valine 5.80 g.
The other ingredients are Water for injections and acetic acid.
Approximate electrolyte concentrations (mmol/l) of the solution:
Acetate (1): 82
Chloride (2): 40
Amino acid concentration: 100 g/l
Nitrogen equivalent: 16.5 g/l
Protein equivalent: 103 g/l
Essential AA/Total AA ratio: 0.45
pH: approximately 6
Osmolality (mOsm/l): 1060
(1) Acetate is added as acetic acid to adjust the pH.
(2) Chloride ions are due to L-lysine hydrochloride.
Appearance and packaging of the product
Synthamin is a clear, particle-free solution. It is presented in Viaflex plastic bags. Each bag is packaged in a protective overpouch.
The bag sizes can be 500 and 1000 ml.
The bags are supplied in cartons, each containing the following quantities:
- 10 bags of 500 ml
- 10 bags of 1000 ml
Not all pack sizes may be marketed.
Marketing authorisation holder
Baxter S.L.
Pouet de Camilo 2, 46394 Ribarroja del Turia (Valencia), Spain.
Manufacturer
Baxter S.A.
Boulevard René Branquart, 80, 7860 Lessines, Belgium.
Date of last revision of this leaflet: March 2020
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals
Posology and method of administration
Posology
The total daily dose of these solutions depends on the patient's metabolic needs and clinical response.
Probably, the best procedure to determine individual nitrogen needs is to determine fluid balance. Daily doses of amino acids of approximately 1.0-1.5 g/kg body weight in adults and 2-3 g/kg body weight in children, with an adequate amount of calories (around 180 kcal/g nitrogen or +/- 30 kcal/g amino acids), are generally sufficient to meet protein needs and promote a positive nitrogen balance.
The use of higher doses, mainly in children, should be monitored through more frequent laboratory tests. The infusion rate should not exceed 0.1 g/kg per hour. The maximum infusion rate depends on the amino acid concentration used. In the case of Synthamin 17 (10% Amino Acids), it will be 70 ml/h.
It is necessary to ensure the maintenance of serum potassium levels. A amount of potassium equivalent to 60-180 mEq/day may be indicated. It may be useful to add electrolytes and potassium, depending on the amount of carbohydrates administered and metabolized by the patient. It is important to frequently monitor serum electrolyte levels, mainly phosphate, magnesium, and chloride. In the case of administration of a solution without electrolytes, the patient should be closely monitored, evaluating their electrolyte needs.
Individually, vitamins, trace elements, and other components (including glucose and lipids) may be added to the parenteral nutrition regimen to meet nutritional needs and prevent deficiencies and the development of complications.
The co-administration of an oily emulsion should be evaluated when prolonged parenteral nutrition is required to prevent essential fatty acid deficiency (EFAD).
In the case of peripheral administration, the osmolality of the solution should be taken into account.
The solution should be visually inspected for particles and color before administration.
The infusion rate of the solution should be gradually increased during the first hour and adjusted according to the dose to be administered, the daily volume indicated, and the duration of the infusion.
The use of a final filter is recommended during the administration of parenteral nutrition solutions.
Method of administration
Hypertonic mixtures of amino acids and glucose are preferably administered through a central catheter. If the central route is not indicated, it can be administered peripherally to patients who need parenteral nutrition, amino acid solutions together with glucose solutions of appropriate concentration, at the same time as lipid emulsions.
Do not administer unless the solution is clear and colorless or slightly yellow and the packaging is intact.
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) should be protected from light exposure until the end of administration.
Special warnings and precautions for use:
Exposure to light of parenteral nutrition solutions for intravenous use, especially after mixing with trace elements or vitamins, may have adverse effects on the clinical outcome of newborns due to the generation of peroxides and other degradation products.
When used in newborns and children under 2 years, Synthamin should be protected from ambient light until the end of administration.
Handling and preparation
Instructions for use of the Viaflex bag
Use an aseptic technique.
- Remove the Viaflex bag from its protective overpouch at the time of use. Discard the oxygen absorber bag.
- Check for absence of leaks by squeezing the Viaflex bag. Check the transparency of the solution and the absence of foreign particles.
- Hang the Viaflex bag. Prepare the administration equipment and close the flow regulator.
- Remove the protector from the outlet tube of the Viaflex bag and the protector from the spike of the administration equipment. Insert the spike of the administration equipment into the outlet tube of the Viaflex bag.
- Follow the instructions for use of the administration equipment to prime and administer the solution.
In the case of additions to the bag:
Use an aseptic technique.
Verify the stability and compatibility of the additives.
Prepare the injection point of the bag.
Puncture the injection point and inject the additives using a syringe or a reconstitution device.
Mix the contents of the bag and the additives.
Inspect the final mixture for color and foreign particles.
Check the bag for absence of leaks.
Ensure that the storage conditions for the additive are met.
Administration of the infusion:
Do not administer blood before, at the same time, or after, through the same equipment due to the risk of pseudoagglutination.
Do not connect bags in series to avoid a gas embolism due to residual air in the primary container.
Discard after single use.
Discard any remaining portion.
Do not reconnect partially used bags.
Special precautions for disposal and other handling:
When used in newborns and children under 2 years, it should be protected from light exposure until the end of administration. Exposure of Synthamin to ambient light, especially after mixing with trace elements or vitamins, generates peroxides and other degradation products that can be reduced if the product is protected from light exposure.
Baxter, Viaflex, and Synthamin are registered trademarks of Baxter International Inc.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SYNTHAMIN 17 REFORMULATED WITHOUT ELECTROLYTES SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for SYNTHAMIN 17 REFORMULATED WITHOUT ELECTROLYTES SOLUTION FOR INFUSION
Discuss questions about SYNTHAMIN 17 REFORMULATED WITHOUT ELECTROLYTES SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















