
SYNAREL 200 micrograms NASAL SPRAY SOLUTION

How to use SYNAREL 200 micrograms NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SYNAREL200 micrograms nasal spray solution
Nafarelin
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet:
- What is Synarel and what is it used for
- What you need to know before you use Synarel
- How to use Synarel
- Possible side effects
- Storage of Synarel
Contents of the pack and other information
1. What is Synarel and what is it used for
Synarel belongs to a class of medicines called synthetic gonadotropin-releasing hormone analogues (a natural substance produced by the body that stimulates the ovaries in women - which are the organs that contain the eggs - and the testicles in men). It works by reducing your body's normal response to this hormone, and as a result, the ovaries produce fewer hormones called estrogens. You will notice this reduction because your menstruation will stop or decrease in amount after about a month of using this medicine.
Synarel is used for:
- Endometriosis (abnormal location of the membrane that lines the uterus), including relief of symptoms that accompany it, such as painful menstruation, painful intercourse, general pelvic pain, and heavy bleeding during menstruation.
- Controlled ovarian stimulation programs before undergoing in vitro fertilization, always under the supervision of a specialist doctor.
2. What you need to know before you use Synarel
Do not use Synarel
- If you are allergic to nafarelin, to natural gonadotropin-releasing hormone, to other gonadotropin-releasing hormone analogue medicines, or to any of the other ingredients of this medicine (listed in section 6).
- If you have vaginal bleeding and the cause is not known.
- If you are pregnant or think you may be pregnant during treatment. If you are using this medicine as part of an in vitro fertilization program, your pregnancy will be planned for when you finish treatment (see section "Pregnancy and breastfeeding").
- If you are breastfeeding.
- If you have already used Synarel for six months (see section "Possible side effects").
Warnings and precautions
Talk to your doctor or pharmacist before you start taking this medicine
- If you forget to take one or more doses, because this could mean a risk of becoming pregnant.
- It is recommended to use non-hormonal barrier contraceptives (diaphragm, intrauterine devices (IUD), condoms) while taking this medicine to avoid becoming pregnant (see section "Pregnancy and breastfeeding").
- If you have a history of multiple cysts in the ovaries, as they may increase in number or size. Although they usually disappear on their own within 4-6 weeks of treatment, in some cases it may be necessary to interrupt treatment and/or undergo surgery.
- If you have a family history of osteoporosis (a disease in which the bones weaken, which can cause pain and increase the risk of bone fractures), as a small loss of bone mineral may occur during treatment (see section "Possible side effects").
- If your menstruation does not decrease after 2 months of treatment. Inform your doctor, as menstruation should stop or decrease in amount during treatment with Synarel. Also, if you do not have normal menstruation 4-8 weeks after stopping treatment with Synarel, consult your doctor.
- If you need to use a nasal decongestant, it should be used at least 30 minutes after using Synarel (see section "Using other medicines").
- If you are going to have tests to explore the hypothalamic-pituitary-gonadal axis, as the results may be altered up to 8 weeks after finishing treatment.
- If, after a treatment period, you need to undergo retreatment, your doctor should determine your bone mineral density before starting to use the medicine again.
- There have been reports of depression in patients taking Synarel, which can be severe. If you are taking Synarel and experience depression, inform your doctor.
- The use of Synarel in combination with gonadotropin to treat infertility can sometimes cause an exaggerated reaction in the ovaries (ovarian hyperstimulation syndrome, OHSS). You may notice stomach pain, stomach swelling, and discomfort or nausea. If this happens, consult your doctor. See also section 4 "Possible side effects".
Children and adolescents
The use of Synarel is not recommended in people under 18 years of age.
Other medicines and Synarel
Tell your doctor or pharmacist if you are using or have recently used or might use other medicines, including those obtained without a prescription.
It is not expected that Synarel will interact with other medicines.
Nasal decongestants reduce the absorption of Synarel, so you should not use this type of medicine in the 30 minutes following administration of Synarel. However, rhinitis (inflammation of the inner lining of the nose due to allergy or a cold, which can clog your nose) does not seem to alter the absorption of Synarel, so you can continue to use Synarel normally (see section "How to use Synarel").
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Before starting treatment with Synarel, it should be confirmed that you are not pregnant. You should not use Synarel if you are pregnant or think you may be pregnant.
During treatment, you should avoid becoming pregnant using non-hormonal barrier contraceptives such as a diaphragm, IUD, condoms, but not oral contraceptives, as this is a hormonal method. This may seem strange if you are taking Synarel as part of an in vitro fertilization program, but this is because your pregnancy will be planned for when you finish treatment and should not occur beforehand. If you do become pregnant during treatment, you should stop treatment and inform your doctor, who will provide more detailed information on this topic.
This medicine should not be administered during breastfeeding.
Driving and using machines
The influence on the ability to drive and use machines is zero or insignificant.
Synarel contains benzalkonium chloride.
This medicine contains 0.01 mg of benzalkonium chloride per spray.
Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used during long-term treatment.
3. How to use Synarel
Only women aged 18 years or older can use Synarel, as clinical experience is limited to these ages.
Follow your doctor's instructions for administering this medicine exactly.
Treatment with Synarel will be carried out under the control and recommendations of your doctor and requires periodic monitoring by your doctor. Your doctor will indicate the duration of your treatment with Synarel. Do not stop treatment before.
Remember to use your medicine.
Synarel should only be administered nasally. As a general rule, the dose of the medicine and the frequency of administration are as follows:
- Endometriosis:treatment should start between day 2 and day 4 of the menstrual cycle. The recommended dose is 2 sprays per day (400 micrograms), divided into two administrations (one spray in one nostril in the morning and one spray in the other nostril at night). In some cases, it may be necessary to increase the dose to 4 sprays per day (800 micrograms); in this case, two sprays will be administered in the morning (one in each nostril) and two sprays at night (one in each nostril). Your doctor will indicate the recommended dose for you.
The maximum recommended treatment duration is 6 months.
- Controlled ovarian stimulation:if you are taking this medicine as part of an in vitro fertilization program, your doctor will indicate when you should start treatment. The recommended dose is 4 sprays per day (800 micrograms), i.e., one spray in both nostrils in the morning and one spray in both nostrils at night. Your doctor may decide to stop treatment if no response is observed.
Normally, the treatment period does not exceed 8 weeks.
Important tips on using Synarel
- The pump should produce a fine spray, which can only happen through rapid and firm pumping. It is normal to see some larger droplets of liquid in the spray. However, if Synarel comes out of the pump as a jet instead of a fine spray, Synarel may not work properly, and you should consult your pharmacist.
- Make sure to clean the tip of the sprayer before and after each use. If you don't, the tip of the sprayer may become clogged, and the correct dose may not be released. Always put the safety clip and plastic cap on the tip of the sprayer after use to prevent clogging.
- The pump is designed to deliver only a certain amount of medicine, regardless of the force used to press the pump.
- Do not attempt to enlarge the hole in the tip of the sprayer. If the hole is enlarged, the pump will release an incorrect dose of Synarel.
Before the first use, follow these instructions:
Before the first use of each Synarel bottle, the pump must be primed. This step only needs to be done once, before you use this medicine for the first time.
- Remove and save the safety clip and cap to expose the nasal sprayer. Hold the bottle upright, placing 2 fingers on the pump's protrusions and your thumb on the base of the bottle.
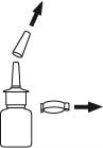
- Prime the pump by pressing the thumb upwards several times firmly and quickly until air comes out and a fine spray appears. This requires 5 to 7 presses, keeping the container with the top end away from you. It is not necessary to repeat this operation with subsequent use of the medicine, as this would waste the medicine.
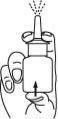
- Clean the spray tip after use:
Hold the bottle horizontally and rinse the spray tip with warm water while cleaning it with your fingers or a soft cloth for 15 seconds.
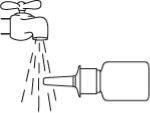
Do not clean the spray tip with a sharp object.This can cause an incorrect amount to be released. Do not separate the pump from the bottle, as this will release the spray pressure.
Dry the spray tip with a soft, clean cloth or a paper towel.
Using the sprayer:
- Blow your nose gently to clear your nostrils.


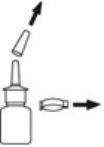
- Remove and save the safety clip and cap from the bottle to expose the nasal sprayer. Hold the bottle as previously shown.
- Clean the nasal spray tip. To do this, hold the bottle horizontally and rinse the spray tip with lukewarm water, cleaning it with your finger or a soft cloth for 15 seconds.
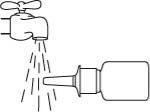
Do not clean the spray tip with a sharp object.This can cause an incorrect amount to be released. Do not separate the pump from the bottle, as this will release the spray pressure.
Dry the spray tip with a soft, clean cloth or a paper towel.
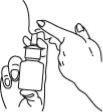
- Tilt your head slightly forward. Insert the spray tip into one nostril, directing it towards the backand outerpart of the nose. Close the other nostril with your finger.
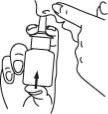
- Press the sprayer firmly once, and breathe in gently at the same time.
- Remove the sprayer from your nose and tilt your head back for a few seconds to allow the liquid to pass to the back of your nose.

- Clean the spray tip. To do this, hold the bottle horizontally and rinse the spray tip with lukewarm water, cleaning it with your finger or a soft cloth for 15 seconds.
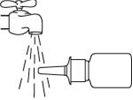
Do not cleanthe spray tipwith a sharp object.This can cause an incorrect amount to be released. Do not separate the pump from the bottle, as this will release the pressure.
Dry the spray tip with a soft, clean cloth or a paper towel.
Clean the spray tip before and after each use to prevent clogging, as clogging could cause an incorrect amount of medicine to be released.
- Put the cap and safety clip on the bottle to cover the spray tip. This is important to prevent the spray tip from becoming clogged.
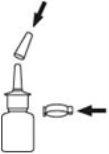
Talk to your doctor or pharmacist if you have any doubts about these instructions.
It is important that you use this medicine regularly, following the recommended doses, daily, in the morning and at night (see section "If you forgot to use Synarel").
Do not stop treatment if you have a common cold. If you want to use a nasal decongestant, it is recommended to use it at least 30 minutes after using Synarel (see section "Using other medicines").
If you sneeze while using this medicine or after using it, it is recommended to repeat the administration. However, if you always sneeze when using Synarel, inform your doctor.
In order to ensure adequate administration of the doses, the bottle should not be used for more than 30 days (2 sprays per day) or 15 days (4 sprays per day); after this time, you will see that a small amount of solution remains in the container, but you should not use it.
If you think the effect of Synarel is too strong or too weak, tell your doctor or pharmacist.
If you use more Synarel than you should, consult your doctor or pharmacist immediately.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone 91-562 04 20.
If you forgot to use Synarel:
You should use this medicine habitually. If you forgot to use it at the right time, do so as soon as you remember, and then use it as indicated, i.e., in the morning and at night.
If you forgot to administer one or more doses, you may experience vaginal bleeding. If you forgot several doses, you may become pregnant. If this happens, you should stop taking this medicine and inform your doctor immediately.
Do not take a double dose to make up for forgotten doses.
4. Possible Adverse Effects
Like all medicines, Synarel can have adverse effects, although not all people suffer from them. The estimated frequency of their appearance in adults is as follows:
The very frequent adverse effects (at least 1 in 10 patients) are:
- Weight gain.
- Affective lability (mood changes), decreased libido (sexual desire).
- Headache.
- Hot flashes.
- Rhinitis (inflammation of the nasal mucosa).
- Acne, seborrhea (excess oil on the skin).
- Myalgia (muscle pain).
- Breast atrophy, vulvovaginal dryness, decreased breast size.
- Edema (swelling).
The frequent adverse effects (at least 1 in 100 patients) are:
- Hypersensitivity to the drug characterized by chest pain, dyspnea (difficulty breathing), pruritus (itching), rash, urticaria (red elevated lesions on the skin).
- Estrogen deficiency (female sex hormones).
- Weight loss.
- Depression, insomnia (difficulty sleeping), increased libido (sexual desire).
- Paresthesia (tingling sensation).
- Hypertension (high blood pressure), hypotension (low blood pressure).
- Nasal mucosa irritation.
- Hirsutism (excessive hair growth).
- Arthralgia (joint pain).
- Artificial menopause, uterine bleeding (bleeding from the uterus).
- Decreased bone density (increased bone porosity).
The infrequent adverse effects (at least 1 in 1,000 patients) are:
- Alopecia (hair loss).
- Increased breast size, ovarian cysts (fluid concentration inside the ovary).
The rare adverse effects (at least 1 in 10,000 patients) are:
- Elevated serum GOT, GPT, and alkaline phosphatase (elevation of liver enzymes, indicators of liver function).
The adverse effects whose frequency is unknown (cannot be estimated from the available data) are:
- Migraine (headache).
- Blurred vision (vision impairment).
- Palpitations (perception of heart activity).
- Ovarian hyperstimulation syndrome (exaggerated response to ovarian stimulation that can cause abdominal distension, nausea and/or vomiting, and diarrhea. In severe cases, fluid in the abdomen).
Other Adverse Effects in Children and Adolescents
The adverse effects and their estimated frequency of appearance in the pediatric population are as follows:
The very frequent adverse effects (at least 1 in 10 patients) are:
- Breast enlargement.
The frequent adverse effects (at least 1 in 100 patients) are:
- Hypersensitivity to the drug characterized by chest pain, dyspnea (difficulty breathing), pruritus (itching), rash, urticaria (red elevated lesions on the skin).
- Affective lability (mood changes).
- Hot flashes.
- Rhinitis.
- Acne, hirsutism (excessive hair growth), seborrhea (excess oil on the skin), abnormal body odor.
The infrequent adverse effects (at least 1 in 1,000 patients) are:
- Vaginal bleeding, vaginal discharge (vaginal flow).
The adverse effects whose frequency is unknown (cannot be estimated from the available data) are:
- Transient increase in pubic hair.
During the first two months of treatment, some vaginal bleeding may occur. Do not worry, this is normal.
A small decrease in the mineral content of the bones may also occur, which recovers during the 6 months following the interruption of treatment. If you have a family history of osteoporosis, you may be more likely to suffer from bone mineral loss during treatment (see section "Be especially careful with Synarel").
Most of these effects are not serious, disappear at the end of treatment, and do not pose a reason for interrupting it.
Some patients have presented symptoms that may indicate an allergy to this medication, such as difficulty breathing, chest pain, rash, skin redness, and itching. If any of these symptoms appear, stop the medication and consult your doctor.
If you consider that any of the adverse effects you suffer from is serious or if you notice any adverse effect not mentioned in this prospectus, inform your doctor or pharmacist.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Synarel
Do not store at a temperature above 30 °C.
Keep the container in a vertical position (standing up).
Do not freeze.
Keep in the outer packaging to protect it from light.
When not using this medication, keep the cap and safety lock on.
Keep out of reach and sight of children.
Do not use Synarel after the expiration date shown on the packaging.
Each Synarel container can be used for a maximum of 30 days, which may be reduced depending on the dosage (see section "How to use Synarel").
Medicines should not be thrown away through the sewers or in the trash. Deposit the containers and medications you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Synarel Composition
- The active ingredient is nafarelin (as acetate). Each spray releases 100 microliters of aqueous solution containing nafarelin acetate equivalent to 200 micrograms of nafarelin base.
- The other components are: sorbitol (E-420), benzalkonium chloride, glacial acetic acid, and purified water.
Product Appearance and Package Contents
Synarel is presented as a nasal spray solution, each container contains an 8 ml bottle of aqueous solution, provided with a dosing valve. Each bottle contains 60 doses.
Marketing Authorization Holder and Manufacturer
Marketing authorization holder:
Pfizer, S.L.
Avda. de Europa, 20-B
Parque Empresarial La Moraleja
28108 Alcobendas (Madrid)
Spain
Manufacturer:
PFIZER SERVICE COMPANY BV
Hoge Wei 10 (Zaventem)
B-1930 – Belgium
Date of the Last Revision of this Prospectus: January 2022
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price122.05 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SYNAREL 200 micrograms NASAL SPRAY SOLUTIONDosage form: INJECTABLE, 0.25 mg/0.5 mlActive substance: ganirelixManufacturer: Gp Pharm S.A.Prescription requiredDosage form: INJECTABLE, 0.25 mgActive substance: cetrorelixManufacturer: Intas Third Party Sales 2005 S.L.Prescription requiredDosage form: INJECTABLE, UnknownActive substance: cetrorelixManufacturer: Merck Europe B.V.Prescription required
Online doctors for SYNAREL 200 micrograms NASAL SPRAY SOLUTION
Discuss questions about SYNAREL 200 micrograms NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






