
SECUFEN 50 micrograms/ml injectable solution

How to use SECUFEN 50 micrograms/ml injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What SECUFEN 50 micrograms/ml, solution for injection EFG is and what it is used for
- What you need to know before you are given SECUFEN 50 micrograms/ml, solution for injection EFG
- How SECUFEN is given
- Possible side effects
- Storage of SECUFEN
- CONTENT OF THE PACKAGE AND ADDITIONAL INFORMATION
Introduction
Package Leaflet: Information for the User
SECUFEN 50 micrograms/ml, solution for injection EFG
Sufentanil (citrated)
|
Contents of the pack:
- What SECUFEN is and what it is used for
- What you need to know before you are given SECUFEN
- How SECUFEN is given
- Possible side effects
- Storage of SECUFEN
- Contents of the pack and other information
1. What SECUFEN 50 micrograms/ml, solution for injection EFG is and what it is used for
SECUFEN contains the active substance sufentanil, which is a potent analgesic (pain reliever)
SECUFEN is used in general anesthesia, in resuscitation and in regional anesthesia to relieve pain (analgesic agent)
Adults
In regional anesthesia, SECUFEN solution for injection (IV or epidural) is injected epidurally
This technique is used in so-called painless childbirth, in general surgery or for post-operative pain
Children
- By intravenous route, SECUFEN solution for injection (IV or epidural) is indicated as an analgesic agent in the induction and/or maintenance of balanced general anesthesia in children over one month of age
- By epidural route, SECUFEN solution for injection (IV or epidural) is indicated for the treatment of pain after general, thoracic or orthopedic surgery in children over one year of age
2. What you need to know before you are given SECUFEN 50 micrograms/ml, solution for injection EFG
Do not use SECUFEN:
- If you are allergic (hypersensitive) to sufentanil citrate or to morphine derivatives, or to any of the other ingredients of SECUFEN listed in section 6
- If you are allergic to morphine-derived medications
- In combination with:
- Certain opioid medications (nalbuphine, buprenorphine), naltrexone (a medication used for addiction or prevention of relapse), or nalmefene (a medication used in certain cases of alcohol dependence) (see section "Other medications and SECUFEN")
- Epidural administration may be contraindicated in certain treatments such as anticoagulant treatment (medications that decrease blood clotting), generalized or localized infection at the injection site, and/or significant bleeding (blood loss)
Talk to your doctor or pharmacist if you have any doubts
Warnings and precautions
Be especially careful with SECUFEN solution for injection (IV or epidural):
- As with other analgesics of the morphine type (pain-relieving medications), the use of SECUFEN solution for injection (IV or epidural) may be accompanied by respiratory depression (acute respiratory failure) that may persist for some time after surgery
- Consequently, you will be under medical supervision for a while after surgery
- If you experience significant drowsiness or respiratory problems after surgery, inform your doctor or healthcare staff immediately
- When SECUFEN solution for injection (IV or epidural) is used for prolonged sedation, you will receive respiratory assistance
- Generally, this medication cannot be administered if you are consuming alcoholic beverages, medications containing alcohol, crizotinib or idelalisib (anticancer medications), or sodium oxybate (see section "Warnings and precautions")
- Tell your doctor if you or a family member has a history of mental illness (such as depression), alcoholism, or addiction, as the risk of dependence on sufentanil may increase depending on the dose and duration of treatment
- The use (even at therapeutic doses) may lead to physical dependence, as it can cause withdrawal effects and the reappearance of problems if the medication is abruptly stopped
- Tell your doctor if you are pregnant, think you may be pregnant, or are breastfeeding (for more information, see the section "Pregnancy")
- Tell your doctor if you experience increased sensitivity to pain, despite taking increasing doses (hyperalgesia). Your doctor will decide if you need to modify the dose or stop taking this medication
Tell your anesthesiologist if you have:
- Low blood pressure, hypovolemia, or heart failure
- Blood flow problems in the brain
- Any chronic respiratory disease
- Liver or kidney problems
- Hormonal deficiency of the thyroid gland
Talk to your doctor if you have any doubts
Children and adolescents
Due to the risk of overdose or underdose, the use of SECUFEN by intravenous route is not recommended during the neonatal period
The use of SECUFEN by epidural route is not recommended in children under one year of age
Other medications and SECUFEN
Tell your doctor or pharmacist if you are using or have recently used any other medications, including those purchased without a prescription, homeopathic, herbal, or other health-related products, as it may be necessary to interrupt treatment or adjust the dose of one of them
This medication cannot be administeredin any casewith certain opioids (nalbuphine, buprenorphine), with naltrexone (medication used for addiction or prevention of relapse), or with nalmefene (medication used in certain cases of alcohol dependence)
This medication cannot, in general,be administered if you are consuming alcoholic beverages, medications containing alcohol, crizotinib or idelalisib (anticancer medications), or sodium oxybate (medication used to treat a certain type of sleep disorder)
You must tell your doctor if you are taking:
Medications that contain:
- Erythromycin, clarithromycin, or telithromycin (antibiotics)
- Itraconazole, voriconazole, posaconazole, or ketoconazole (to treat infections caused by microscopic fungi)
- Nelfinavir or ritonavir (used to treat HIV infection)
·Strong analgesics or sedative medications (for example, medications used to treat sleep disorders, medications used to reduce anxiety, medications for mental disorders, some cough medications), as the dose of SECUFEN may need to be reduced
Similarly, if you are given a strong analgesic or another sedative medication after receiving SECUFEN during surgery, it may be necessary to reduce the dose of the analgesic or sedative medication to reduce the risk of serious side effects, such as respiratory problems, slow or shallow breathing, intense drowsiness, and decreased consciousness, coma, or death
·Medications used to treat depression, such as monoamine oxidase inhibitors (MAOIs). These medications should not be taken within two weeks prior to the administration of SECUFEN, or at the same time
·Medications used to treat depression, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). It is not recommended to take these medications at the same time as SECUFEN
Tell your doctor or pharmacist if you are using or have recently used any other medication
Using SECUFEN with food and drinks:
In order to administer this medication to you, you should avoid consuming alcoholic beverages (see section "Warnings and precautions")
Pregnancy and breastfeeding:
Tell your anesthesiologist if you are pregnant or breastfeeding
SECUFEN should not be used during pregnancy, unless it is strictly necessary
When this medication is administered to women during pregnancy, there is a risk that the newborn may present with neonatal withdrawal syndrome and respiratory depression
The administration of SECUFEN in breastfeeding women should be done with caution and with doses not exceeding 30 µg; it is recommended to wait 4 hours after the administration of sufentanil before breastfeeding. Breastfeeding is not recommended with higher doses
Driving and using machines:
SECUFEN may decrease your alertness or ability to drive
A reasonable time should pass (at least 24 hours) between the administration of this medication and the resumption of driving or using machines
Always consult your doctor
The concomitant use of SECUFEN and sedative medications such as benzodiazepines or related medications (which can relieve anxiety and convulsions, allow muscle relaxation, and promote sleep) increases the risk of drowsiness, difficulty breathing, depression, and coma, and is likely to cause death. Therefore, the concomitant use of these medications should only be considered in the absence of any other viable treatment option. However, if SECUFEN is prescribed simultaneously with benzodiazepines and/or related medications, your doctor should limit the dose and duration of these concomitant treatments. Inform your doctor about all medications you are taking and follow their dosing recommendations strictly. It may be helpful to inform your friends or family members so they are aware of the signs and symptoms mentioned above. Contact your doctor if you notice these symptoms
Important information about some of the ingredients of SECUFEN
Ampoules of 2 ml
This medication contains less than 23 mg of sodium per ampoule, i.e., it is essentially "sodium-free"
Ampoules of 10 ml:
Patients on low-sodium diets should note that this medication contains 35.40 mg (1.5 mmol) of sodium per ampoule
3. How SECUFEN is given
This medication will be administered exclusively by specially trained personnel in anesthesia-resuscitation or emergency medicine, who are familiar with the use of anesthetics, or under their control, and in fully equipped locations for the control and assistance of respiratory and cardiovascular functions
Posology
The dose, administered by a healthcare professional, will be determined based on your:
- age
- weight
- general condition
- type of anesthesia used
Method of administration and route of administration
This medication will be administered to you by intravenous route(in a vein) to relieve pain throughout the body during surgical interventions or by epidural route(in the lower back), to relieve pain in some parts of the body, for example during childbirth or after an intervention
If you think you have been given too much SECUFEN
This medication will only be administered to you in a hospital by healthcare professionals, so it is unlikely that you will receive more SECUFEN than you should; however, if you think you have been given too much SECUFEN, inform your doctor immediately. In case of accidental overdose, call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount administered
It is recommended to take the packaging and the package leaflet of the medication to the healthcare professional
If you miss a dose of SECUFEN
This medication will only be administered to you in a hospital by healthcare professionals, so it is unlikely that you will miss a dose of SECUFEN; however, if you think you have missed a dose of this medication, inform your doctor immediately
If you have any other questions about the use of this product, ask your doctor, pharmacist, or nurse
4. Possible side effects
Like all medications, SECUFEN can have side effects, although not everyone gets them
Frequencies are defined as very common (≥ 1/10), common (≥ 1/100 to <1>
Very common side effects(may affect more than 1 in 10 people)
- Sedation
- Itching
Common side effects(may affect 1 to 10 people in 100)
- Newborn tremors
- Dizziness
- Headache
- Increased heart rate
- Hypertension
- Hypotension
- Pallor
- Bluish discoloration of the skin (nails and lips) of the newborn
- Vomiting
- Nausea
- Discoloration of the skin
- Muscle contractions
- Urinary retention
- Urinary incontinence (involuntary loss of urine)
- Fever
Uncommon side effects(may affect 1 to 10 people in 1,000)
- Cold
- Allergy
- Apathy
- Nervousness
- Difficulty coordinating movements
- Abnormal movements of the newborn
- Involuntary and painful muscle contractions
- Exaggerated reflexes
- Increased muscle tone
- Decreased motor activity of the newborn
- Drowsiness
- Vision disorders
- Bluish discoloration of the skin (nails and lips)
- Heart rhythm disorder
- Slow heart rate
- Irregularities of heart contractions
- Abnormal electrocardiogram
- Breathing difficulties
- Decreased lung ventilation
- Voice disorders
- Cough
- Hiccup
- Respiratory disorder
- Allergic skin inflammation
- Excessive sweating
- Widespread skin rash (also in newborns)
- Dry skin
- Lower back pain
- Decreased muscle tone in the newborn
- Muscle stiffness
- Increased or decreased body temperature
- Chills
- Pain
- Reaction at the injection site
- Pain at the injection site
Side effects of unknown frequency(cannot be estimated from available data)
- Allergic reactions
- Coma
- Seizure
- Involuntary muscle contractions
- Pupil constriction
- Cardiac arrest
- Acute distress with a drop in blood pressure
- Respiratory arrest
- Apanea
- Respiratory depression
- Pulmonary edema
- Sudden contraction of the laryngeal muscles
- Redness of the skin
- Muscle spasms
Children and adolescents
It is expected that the frequency, type, and severity of side effects will be the same as in adults
If you think any of the side effects are serious or if you notice any side effects not mentioned in this leaflet, tell your doctor or pharmacist
Reporting of suspected adverse reactions
It is important to report any suspected adverse reactions to the medication after its authorization. This allows for continuous monitoring of the benefit-risk ratio of the medication. Healthcare professionals are invited to report any suspected adverse reactions through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es
5. Storage of SECUFEN
Keep this medication out of the sight and reach of children
Before dilution: Store the ampoules in the original packaging, protected from light
After dilution: From a microbiological point of view, the product should be used immediately
Do not use SECUFEN after the expiration date stated on the packaging. The expiration date is the last day of the month indicated
Medications should not be disposed of through wastewater or household waste. Ask your doctor how to dispose of the packaging and any unused medication. This will help protect the environment
6. CONTENT OF THE PACKAGE AND ADDITIONAL INFORMATION
Composition ofSECUFEN
- The active ingredient is sufentanil (in the form of citrate).
SECUFEN 50 micrograms/ml:
Each ml of solution contains 50 micrograms of sufentanil in the form of sufentanil citrate. The other components are: Sodium chloride, sodium hydroxide (to adjust the pH), hydrochloric acid (to adjust the pH) and water for injectable preparations.
.
Appearance of the product and package content:
SECUFEN is an injectable solution presented in sterile ampoules of 5 ml. Each package contains 10 ampoules of 5 ml.
An ampoule of 5 ml contains 250 micrograms of sufentanil (citrato).
Marketing authorization holder:
Altan Pharmaceuticals, S.A.
C/ Cólquide, 6 Portal 2, 1ª Planta, Oficina F
Edificio Prisma
28230 Las Rozas (Madrid) -Spain
Manufacturer:
LABORATOIRE RENAUDIN
Z.A. Errobi
64250 ITXASSOU (France)
Date of the last revision of this prospectusMay 2024
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)http://www.aemps.gob.es
This information is intended only for doctors or healthcare professionals
Special warnings
At the beginning of treatment, this medication may induce muscle stiffness.
This stiffness can be avoided if the following measures are taken:
- The injection should be slow enough when using sufentanil in low doses,
- The administration of muscle relaxants immediately before the administration of this medication prevents muscle stiffness.
When used in obstetrics by intravenous route, sufentanil will be administered after clamping the umbilical cord to prevent any depressive effect on the neonate's respiration. However, intravenous use is contraindicated during labor or before clamping the umbilical cord.
Posology and method of administration
This medication will be administered exclusively by specialist doctors in anesthesia - resuscitation or emergency medicine, familiar with the use of anesthetics, or under their control, and who have the necessary anesthesia - resuscitation equipment.
Information intended for the person in charge of administering SECUFEN injectable solution (IV or epidural route):
The posology varies according to the anesthesia technique, the patient's condition, and the ventilation control modalities.
Depending on the different indications, the method of use, and the posologies are as follows:
Adults
Intravenous route
Balanced general anesthesia
Interventions of short or medium duration (one or two hours): 0.1 to 2 micrograms/kg for induction associated with a hypnotic and/or a volatile anesthetic agent and a muscle relaxant.
Doses of 10 to 25 micrograms of sufentanil may be administered for the maintenance of anesthesia, depending on the clinical signs of decreased analgesia and tolerance to the initial dose.
Major surgical interventions (more than 2 hours): the total dose will be calculated based on the administration of 1 microgram/kg/hour, to be adapted according to the surgical intervention, the patient's condition, and the associated products. 75% of the total dose can be administered as a bolus for induction and maintenance can be ensured either by injections of 10 to 50 micrograms depending on the clinical signs of decreased analgesia or by continuous perfusion. Sufentanil can be associated with a hypnotic and/or a volatile anesthetic agent and a muscle relaxant.
Analgesic anesthesia (cardiovascular surgery)
A bolus dose of 8 to 20 micrograms/kg will be administered for induction associated with 100% oxygen and a muscle relaxant compatible with the patient's cardiovascular status.
A supplementary bolus of 5 to 10 micrograms/kg should be administered before sternotomy.
Maintenance should be ensured, either with repeated doses of 25 to 50 micrograms administered according to the signs of decreased analgesia and the patient's tolerance to the initial bolus, or by continuous perfusion.
Compared to other morphine derivatives used in these protocols, the doses of associated medications such as volatile anesthetics, benzodiazepines, should generally be reduced.
The average total dose administered in cardiovascular surgery is 12 to 30 micrograms/kg, with an average extubation period of 12 to 18 hours.
However, the posology should be adjusted according to the other anesthetic agents used, as well as individual variations and the extubation period.
Prolonged sedation in intensive care or resuscitation units for ventilated patients
From 0.2 to 2 micrograms/kg/hour, depending on the degree of sedation required and the doses of products eventually associated.
Epidural route
General surgery (thoracic, urologic, orthopedic)
An initial dose of 0.75 micrograms/kg, diluted in 10 ml, allows analgesia of 4 to 8 hours. Supplementary boluses of 25 to 50 micrograms can be administered according to the signs of decreased analgesia.
Obstetrics
Bolus doses of 15 to 20 micrograms diluted in a volume of 10 ml, associated with a local anesthetic such as bupivacaine (0.125%-0.25%). It is recommended not to exceed the total dose of 30 micrograms of sufentanil.
Post-cesarean analgesia
Bolus doses of 25 µg diluted in a volume of 10 ml, associated with a local anesthetic such as bupivacaine (0.125%-0.25%). It is recommended not to exceed the total dose of 30 micrograms of sufentanil.
Postoperative analgesia
Bolus doses of 0.75 micrograms/kg diluted in a volume of 10 ml, in a single dose or repeated according to the signs of decreased analgesia (25 to 50 micrograms), or in perfusion at a rate of 0.2 to 0.3 micrograms/kg/hour.
Pediatric population
Intravenous administration
Due to the great variability of pharmacokinetic parameters in neonates, no posological recommendation can be given (see sections 4.4 and 5.2).
Children over one month
Regardless of the dose, premedication with an anticholinergic such as atropine is recommended, unless contraindicated.
Induction of anesthesia
SECUFEN can be administered in a slow bolus over at least 30 seconds at a dose of 0.2 to 0.5 micrograms/kg, in combination with another anesthetic agent for induction. In the case of major surgery (e.g., cardiac surgery), doses of up to 1 microgram/kg can be administered.
Maintenance of anesthesia in ventilated patients
In balanced general anesthesia, the dose depends on the dose of the associated anesthetic agents and the type and duration of the surgery. An initial dose of 0.3 to 2 micrograms/kg administered in a slow bolus over at least 30 seconds can be followed by additional boluses of 0.1 to 1 microgram/kg as needed, up to a total dose of 5 micrograms/kg during cardiac surgery.
Epidural route
SECUFEN should only be administered by the epidural route to children by anesthetists specially trained in pediatric epidural anesthesia and in the management of the respiratory depressive effects of opioids. The necessary resuscitation equipment, including intubation equipment and antimorphines, should be readily available.
After the epidural administration of SECUFEN in children, signs of respiratory depression should be monitored for at least 2 hours.
The use of SECUFEN by the epidural route in children has only been documented in a small number of cases.
Children under one year
The safety and efficacy of SECUFEN have not yet been established in children under one year (see sections 4.4 and 5.1).
The data currently available in children over 3 months are described in section 5.1, but no recommendations can be made.
No data are available for neonates and infants under 3 months.
Children over one year
A single bolus dose of 0.25 to 0.75 micrograms/kg administered during surgery provides an analgesic effect for 1 to 12 hours. The duration of the analgesic effect depends on the type of surgical intervention and the concomitant use of a local epidural anesthetic of the amide type.
.
METHOD OF USE
If necessary, SECUFEN, injectable solution (IV or epidural route), can be mixed with saline or glucose solutions. These dilutions are compatible with plastic bags for perfusion. The dilutions should be used within 24 hours of preparation.
Instructions for opening the ampoules
Protective gloves should be used when opening the ampoules.

- Hold the ampoule between the index and thumb fingers, allowing the tip of the ampoule to protrude.

- With the other hand, hold the top of the ampoule with the index finger against the neck of the ampoule and the thumb over the colored point, parallel to the colored ring(s).
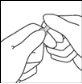
- Maintaining the thumb on the point, break the top of the ampoule with a sharp movement while holding the body of the ampoule firmly in the hand.
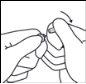
Any accidental skin exposure should be rinsed with clean water. Avoid using soap, alcohol, or any other detergent that may cause chemical or physical abrasion of the skin.
A single bolus dose of 0.25 to 0.75 micrograms/kg administered during surgery provides an analgesic effect for 1 to 12 hours. The duration of the analgesic effect depends on the type of surgical intervention and the concomitant use of a local epidural anesthetic of the amide type.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SECUFEN 50 micrograms/ml injectable solutionDosage form: SUBLINGUAL TABLET, 30 MICROGRAMSActive substance: sufentanilManufacturer: Laboratoire AguettantPrescription requiredDosage form: INJECTABLE, 5 micrograms/mlActive substance: sufentanilManufacturer: Medochemie LimitedPrescription requiredDosage form: INJECTABLE, 50 micrograms/mlActive substance: sufentanilManufacturer: Medochemie LimitedPrescription required
Online doctors for SECUFEN 50 micrograms/ml injectable solution
Discuss questions about SECUFEN 50 micrograms/ml injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















