
Sufentanil Kalceks
Ask a doctor about a prescription for Sufentanil Kalceks

How to use Sufentanil Kalceks
Leaflet accompanying the packaging: information for the user
Sufentanil Kalceks, 5 micrograms/mL, solution for injection/infusion
Sufentanil Kalceks, 50 micrograms/mL, solution for injection/infusion
Sufentanil
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Sufentanil Kalceks and what is it used for
- 2. Important information before using Sufentanil Kalceks
- 3. How to use Sufentanil Kalceks
- 4. Possible side effects
- 5. How to store Sufentanil Kalceks
- 6. Contents of the packaging and other information
1. What is Sufentanil Kalceks and what is it used for
Sufentanil Kalceks contains the active substance sufentanil. It belongs to a group of medicines called "opioid anesthetics". Sufentanil is used as a pain reliever during or after surgery or to reduce pain during childbirth. Sufentanil is used as an anesthetic during surgical procedures in ventilated patients.
Adults
Intravenously (into a vein):
- to prevent pain during the induction and maintenance of general anesthesia in combination with other anesthetic medicines;
- as a medicine for the induction and maintenance of general anesthesia during major surgical procedures.
Epidurally (into the spine):
- to prevent pain after surgery and after cesarean section;
- to treat pain during childbirth.
Children
Sufentanil administered intravenouslyis indicated as a pain reliever for use during the induction and/or maintenance of general anesthesia in children over 1 month of age. Sufentanil administered epidurallyis indicated for the treatment of postoperative pain after general surgery, thoracic surgery, or orthopedic surgery in children over 1 year of age.
2. Important information before using Sufentanil Kalceks
When not to use Sufentanil Kalceks
- if the patient is allergic to sufentanil or any of the other ingredients of this medicine (listed in section 6);
- if the patient has any disease that causes breathing difficulties (e.g. asthma or chronic bronchitis);
- if the patient has a liver enzyme disease called "acute hepatic porphyria";
- if the patient is taking any medicines used to treat depression, known as monoamine oxidase inhibitors (MAOI). MAOI treatment should be stopped 2 weeks before surgery (see "Sufentanil Kalceks and other medicines");
- if the patient is taking or has recently taken other strong painkillers (e.g. nalbuphine, buprenorphine, pentazocine) (see "Sufentanil Kalceks and other medicines").
- Intravenously
- if the patient is in labor or before the umbilical cord is clamped during a cesarean section.
- Epidurally
- if the patient has severe bleeding or shock;
- if the patient has a severe infection;
- if the patient has impaired wound healing;
- if the patient has an infection at the injection site;
- if the patient has changes in blood cell count or is being treated with anticoagulant medicines.
Warnings and precautions
Sufentanil Kalceks should only be administered by trained anesthesiologists in hospitals or other facilities equipped with ventilatory support and postoperative monitoring equipment. Before starting treatment with Sufentanil Kalceks, you should discuss it with your doctor or nurse if:
- the patient has abnormally slow gut movement;
- the patient has gallbladder or pancreas disease;
- the patient or any of their relatives have ever abused alcohol, prescription drugs, or narcotics ("addiction");
- the patient smokes;
- the patient has ever had mood problems (depression, anxiety, or personality disorder) or has been treated by a psychiatrist for other mental illnesses.
This medicine contains sufentanil, which is an opioid medicine. Repeated use of opioid painkillers can lead to the medicine becoming less effective (the patient becomes accustomed to it). This can also lead to dependence and abuse of the medicine, which can result in life-threatening overdose. If the patient is concerned about the possibility of becoming dependent on Sufentanil Kalceks, it is essential to consult a doctor. During treatment with Sufentanil Kalceks, you should consult a doctor if:
- the patient experiences pain or increased sensitivity to pain (hyperalgesia) that does not respond to a higher dose of the prescribed medicine.
During treatment with this medicine, as with all strong painkillers of this type, breathing rate may decrease. This can last until the recovery period or occur again during this time. Therefore, the patient's condition will be closely monitored after surgery. Sufentanil Kalceks may cause sleep-related breathing disorders, such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms of these disorders may include pauses in breathing during sleep, nighttime awakenings due to shortness of breath, difficulty staying asleep, or excessive daytime sleepiness. If these symptoms are observed, you should contact a doctor. The doctor may consider reducing the dose. This medicine will not be used in patients with myasthenia (a chronic muscle disease), as it may cause muscle stiffness after intravenous administration. Involuntary muscle contractions may occur. This medicine should be used with caution:
- in patients with lung, liver, kidney, or thyroid disease;
- in patients with increased intracranial pressure or head or brain injuries;
- in patients with decreased blood volume (administration of this medicine may cause low blood pressure and slow heart rate);
- in elderly and debilitated patients (the dose may need to be reduced). Close medical supervision may be required.
Newborns/Infants
Newborns, like other opioids, are sensitive to respiratory difficulties after sufentanil administration. Only limited data on sufentanil are available after intravenous administration in infants. Therefore, the doctor will carefully assess the benefits and risks before using this medicine in newborns and infants. Due to the risk of overdose or underdose, it is not recommended to use sufentanil intravenously in the neonatal period. Sufentanil should not be used epidurally in children under 1 year of age.
Sufentanil Kalceks and other medicines
Tell your doctor or nurse about all medicines you are taking or have recently taken, as well as any medicines you plan to take. It is especially important if you are taking or have recently taken any of the following medicines:
- strong painkillers, such as opioids (e.g. nalbuphine, buprenorphine, pentazocine) (see "When not to use Sufentanil Kalceks");
- medicines used to treat depression, known as monoamine oxidase inhibitors (MAOI). These medicines should not be taken within 2 weeks before Sufentanil Kalceks administration or at the same time as Sufentanil Kalceks (see "When not to use Sufentanil Kalceks").
- medicines used to treat depression, known as selective serotonin reuptake inhibitors (SSRI) and serotonin-norepinephrine reuptake inhibitors (SNRI). It is not recommended to use these medicines at the same time as Sufentanil Kalceks.
- sedatives and anxiolytics, such as barbiturates, sedatives, or benzodiazepines (e.g. diazepam, midazolam);
- muscle relaxants (e.g. vecuronium, suxamethonium);
- medicines used for general anesthesia (e.g. thiopental, etomidate, nitrous oxide);
- medicines used to treat mental disorders (antipsychotics);
- antibiotics used to treat bacterial infections (erythromycin);
- medicines used to treat fungal infections (e.g. ketoconazole, itraconazole);
- medicines used to treat viral infections (e.g. ritonavir used to treat HIV/AIDS).
Concomitant use of opioids and medicines used to treat epilepsy, nerve pain, or anxiety (gabapentin and pregabalin) increases the risk of opioid overdose and respiratory depression, which can be life-threatening. Concomitant use of Sufentanil Kalceks and sedatives, such as benzodiazepines or related medicines, increases the risk of sedation, breathing difficulties (respiratory depression), coma, and can be life-threatening. Therefore, their concomitant use should only be considered when other treatment options are not possible. If the doctor prescribes Sufentanil Kalceks with sedatives, the dose and duration of concomitant treatment should be determined by the doctor. You should inform the doctor about all sedatives you are taking and strictly follow the doctor's instructions for dosing. It may be helpful to inform friends or relatives to be aware of the signs and symptoms mentioned above. If such symptoms occur, you should contact a doctor.
Sufentanil Kalceks with alcohol
Alcohol may enhance some of the side effects of sufentanil. Therefore, you should not drink alcohol before and after using this medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or nurse for advice before using this medicine. Sufentanil Kalceks should not be administered intravenously during labor, as it crosses the placenta and may affect the baby's breathing. Sufentanil Kalceks may be administered epidurally during labor. Sufentanil passes into breast milk. Caution should be exercised when sufentanil is administered to a breastfeeding woman. Breastfeeding can be started 24 hours after the last use of sufentanil.
Driving and using machines
This medicine can have a major impact on your ability to drive and use machines. You should not drive or operate machinery until an appropriate amount of time has passed after receiving this medicine. You should leave the hospital with a companion and avoid drinking alcohol.
Sufentanil Kalceks contains sodium
The medicine contains 3.54 mg of sodium (the main component of common salt) per mL of solution. This corresponds to 0.18% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to use Sufentanil Kalceks
Sufentanil Kalceks will be injected by a trained doctor into a vein (intravenously) or into the space surrounding the spinal cord (epidurally). During treatment with Sufentanil Kalceks, specially trained healthcare professionals will closely monitor the patient, and emergency equipment will be available if needed.
Dosage
The doctor will decide what dose and how long the patient (or child) will receive this medicine. The dose depends on age, weight, and physical condition, the type of surgical procedure, and the depth of anesthesia.
- The dose should be carefully adjusted in patients with hypothyroidism, impaired lung function, obesity, and alcoholism. After surgery, it is recommended to monitor these patients for a longer period.
- Patients with liver or kidney disease will require lower doses.
- Elderly and debilitated patients will require lower doses.
Use in children over 1 month and adolescents
Intravenous administration
Sufentanil is injected slowly into a vein by an anesthesiologist. The dosage depends on the dose of other anesthetics, the type and duration of the procedure, and will be determined by the anesthesiologist.
Use in children over 1 year and adolescents
Epidural administration
Sufentanil is injected slowly into the epidural space (part of the spine) by an anesthesiologist experienced in pediatric anesthesia techniques. The dosage depends on the concomitant use of local anesthetics and the required duration of analgesia. Children will be monitored for signs of abnormally slow and shallow breathing (respiratory depression) for at least 2 hours after epidural administration of sufentanil.
Using a higher dose of Sufentanil Kalceks than recommended
Since this medicine will usually be administered by a doctor in carefully controlled conditions, it is unlikely that too much of it will be given. However, if the patient accidentally receives too much sufentanil, breathing difficulties (weak or slow breathing or even temporary cessation of breathing) may occur. In this case, you should immediately inform the doctor or nurse. If you have any further questions about using this medicine, ask your doctor or pharmacist. The medicine should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The most common side effects are excessive sedation, itching, nausea, and vomiting. You should immediatelyinform your doctor or nurse if you experience the following side effects (frequency cannot be estimated from the available data):
- breathing difficulties
- severe allergic reactions, which can cause skin rash, swelling of the face, lips, tongue, or throat, breathing difficulties, loss of consciousness, and anaphylactic shock
Very common(may affect more than 1 in 10 people)
- excessive sedation
- itching
Common(may affect up to 1 in 10 people)
- dizziness, headache
- rapid heartbeat
- high or low blood pressure, paleness
- nausea, vomiting
- skin discoloration
- muscle twitching
- urinary retention or difficulty urinating
- fever
- in newborns: involuntary muscle twitching, blue-tinged skin due to low oxygen levels in the blood
Uncommon(may affect up to 1 in 100 people)
- runny nose or stuffy nose (rhinitis)
- hypersensitivity
- apathy, nervousness
- muscle twitching (intraoperative muscle movements), lack of voluntary muscle coordination, persistent muscle contractions causing twisting and repetitive movements, hyperactive reflexes, abnormal increase in muscle tone, drowsiness
- vision disturbances
- heart rhythm disturbances (atrioventricular block), blue-tinged skin due to low oxygen levels in the blood, slow heart rate, irregular heartbeat, abnormal electrocardiogram, lack of heartbeat
- bronchospasm, too shallow or too slow breathing, voice weakness, cough, hiccups, breathing difficulties
- allergic skin reaction, abnormal sweating, rash, dry skin
- back pain, muscle stiffness, including chest wall stiffness that can cause breathing difficulties
- chills, elevated or decreased body temperature, reaction at the injection site, pain at the injection site, pain
- in newborns: involuntary muscle twitching, decreased body movements, rash, decreased muscle tone
Frequency not known(frequency cannot be estimated from the available data)
- involuntary muscle contractions, overwhelming feeling of well-being (euphoria), feeling of "spinning" (dizziness), coma, seizures
- constricted pupils
- cardiac arrest (the doctor has medicines to reverse this effect)
- shock
- respiratory arrest, water in the lungs, laryngospasm
- skin redness
- muscle contractions
Side effects in children and adolescents
It is expected that the frequency, type, and severity of side effects in children are the same as in adults.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or nurse. Side effects can be reported directly to the Department of Medicinal Product Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Sufentanil Kalceks
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the ampoule label after "EXP" and on the carton after "Expiry date (EXP)". The expiry date refers to the last day of the month stated. There are no special storage instructions for this medicine. Shelf-life after opening of the ampoule The medicine should be used immediately. Shelf-life after dilution Chemical and physical stability has been demonstrated for 72 hours at 20-25°C and 2-8°C. From a microbiological point of view, the diluted solution should be used immediately. If the solution is not used immediately, the responsibility for the storage time and conditions before use lies with the user, and it is normally not longer than 24 hours at 2-8°C, unless dilution has taken place in controlled and validated aseptic conditions. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Sufentanil Kalceks contains
- The active substance is sufentanil (as sufentanil citrate).
Sufentanil Kalceks 5 micrograms/mL
Each mL of solution contains 5 micrograms of sufentanil as sufentanil citrate.
Each 2 mL of solution contains 10 micrograms of sufentanil as sufentanil citrate.
Each 10 mL of solution contains 50 micrograms of sufentanil as sufentanil citrate.
Sufentanil Kalceks 50 micrograms/mL
Each mL of solution contains 50 micrograms of sufentanil as sufentanil citrate.
Each 5 mL of solution contains 250 micrograms of sufentanil as sufentanil citrate.
Each 10 mL of solution contains 500 micrograms of sufentanil as sufentanil citrate.
Each 20 mL of solution contains 1000 micrograms of sufentanil as sufentanil citrate.
The other ingredients are: sodium chloride, citric acid monohydrate (for pH adjustment), water for injections
- The other ingredients are: sodium chloride, citric acid monohydrate (for pH adjustment), water for injections
What Sufentanil Kalceks looks like and contents of the pack
Clear, colorless solution, free from visible particles.
Ampoules of colorless glass type I with a capacity of 2 mL, 5 mL, 10 mL, or 20 mL with one break point. The ampoules are packed in a protective cover. The protective covers are packed in cardboard boxes. Pack sizes: Sufentanil Kalceks 5 micrograms/mL
5 or 10 ampoules of 2 mL
5 or 10 ampoules of 10 mL
Sufentanil Kalceks 50 micrograms/mL
5 or 10 ampoules of 5 mL
5 or 10 ampoules of 10 mL
5 or 10 ampoules of 20 mL
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
AS KALCEKS
Krustpils iela 71E
LV-1057 Rīga
Latvia
Tel.: +371 67083320
Email: [email protected]
This medicine is authorized in the Member States of the European Economic Area under the following names:
Denmark, Czech Republic, Italy, Poland, Slovakia
Sufentanil Kalceks
Austria
Sufentanil Kalceks 5 Mikrogramm/ml, 50 Mikrogramm/ml Injektions-/Infusionslösung
Belgium
Sufentanil Kalceks 5 microgrammes/ml, 50 microgrammes/ml, solution injectable/pour perfusion
Sufentanil Kalceks 5 microgram/ml, 50 microgram/ml oplossing voor injectie/infusie
Sufentanil Kalceks 5 Mikrogramm/ml, 50 Mikrogramm/ml Injektions-/Infusionslösung
France
SUFENTANIL KALCEKS 5 microgrammes/ml, solution injectable/pour perfusion
SUFENTANIL KALCEKS 50 microgrammes/ml, solution injectable/pour perfusion
Germany
Sufentanil Kalceks 5 Mikrogramm/ml Injektions-/Infusionslösung
Sufentanil Kalceks 50 Mikrogramm/ml Injektions-/Infusionslösung
Latvia
Sufentanil Kalceks 5 mikrogrami/ml, 50 mikrogrami/ml šķīdums injekcijām/infūzijām
Netherlands
Sufentanil Kalceks 5 microgram/ml, 50 microgram/ml oplossing voor injectie/infusie
Date of last revision of the leaflet: 04/2023
------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Incompatibilities
This medicinal product must not be mixed with other medicinal products, except those mentioned below.
Special precautions for disposal and preparation of the medicinal product prior to administration
Sufentanil should only be administered by anesthesiologists or doctors familiar with its use and action or under their control. Epidural administration must be performed by a doctor experienced in epidural administration techniques. Before administration, the correct placement of the needle or catheter should be checked. For single use only. All unused solution should be discarded. This medicinal product should be inspected before use. It should not be used if signs of deterioration are visible (e.g. solid particles or discoloration). It can be diluted with:
- 9 mg/mL (0.9%) sodium chloride solution for injection
- 50 mg/mL (5%) glucose solution for injection
- Ringer's solution
- Ringer's solution with lactate
For epidural administration, the medicinal product can be mixed with 9 mg/mL (0.9%) sodium chloride solution and/or bupivacaine solution. Instructions for opening the ampoule
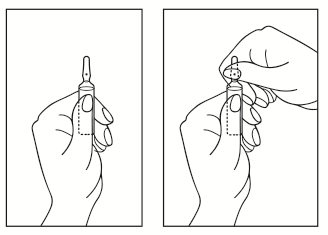
- 1) Turn the ampoule with the colored break point facing upwards. If there is solution in the upper part of the ampoule, gently tap with your finger to move all the solution to the lower part of the ampoule.
- 2) Use both hands to open the ampoule; holding the lower part of the ampoule in one hand, break the upper part of the ampoule in the direction of the colored break point (see picture below).
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAS Kalceks
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sufentanil KalceksDosage form: Solution, 5 mcg/ml (50 mcg/10 ml)Active substance: sufentanilPrescription not requiredDosage form: Solution, 50 mcg/ml, (250 mcg/5 ml, 1 mg/20 ml)Active substance: sufentanilPrescription not requiredDosage form: Solution, 5 mcg/mlActive substance: sufentanilManufacturer: hameln rds a.s. HBM Pharma s.r.o. Siegfried Hameln GmbHPrescription not required
Alternatives to Sufentanil Kalceks in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sufentanil Kalceks in Ukraine
Alternative to Sufentanil Kalceks in Spain
Online doctors for Sufentanil Kalceks
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sufentanil Kalceks – subject to medical assessment and local rules.














