STRIVERDI RESPIMAT 2.5 micrograms solution for inhalation

How to use STRIVERDI RESPIMAT 2.5 micrograms solution for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for theuser
Striverdi Respimat 2.5 micrograms, inhalation solution
olodaterol
Read the entire package leaflet carefully before starting totake this medication, as it contains important information for you.
-Keep this package leaflet, as you may need to read it again.
-If you have any questions, consult your doctor or pharmacist.
-This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms, as it may harm them.
-If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Striverdi Respimat and what is it used for
- What you need to know before taking Striverdi Respimat
- How to take Striverdi Respimat
- Possible side effects
- Storage of Striverdi Respimat
- Package contents and additional information
1. What is Striverdi Respimat and what is it used for
Striverdi Respimat helps people with chronic obstructive pulmonary disease (COPD) breathe more easily. COPD is a long-term lung disease that causes difficulty breathing and coughing. The term COPD is associated with chronic bronchitis and emphysema. Since COPD is a long-term disease, you should use Striverdi Respimat every day and not just when you have breathing problems or other COPD symptoms.
Striverdi Respimat contains the active ingredient olodaterol, which is a long-acting bronchodilator (long-acting beta 2 agonist) that helps open your airways and makes it easier to take and expel air from your lungs. Regular use of Striverdi Respimat can also help you when you have persistent difficulty breathing due to your disease and will help minimize the effects of the disease on your daily life.
2. What you need to know before taking Striverdi Respimat
Do not take Striverdi Respimat
- if you are allergic to olodaterol or any of the other ingredients of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to take Striverdi Respimat
- if you have asthma (you should not take Striverdi Respimat for the treatment of asthma) if you have heart problems
- if you have high blood pressure (hypertension)
- if you have epilepsy
- if you have a specific thyroid gland problem called thyrotoxicosis
- if you have an abnormal enlargement of an artery called an aneurysm
- if you have diabetes
- if you have severe liver failure, as Striverdi Respimat has not been studied in this type of patient
- if you have severe kidney failure, as experience with Striverdi Respimat in these patients is limited
- if you are scheduled for surgery
During treatment with Striverdi Respimat
- Stop taking the medication and consult your doctor immediatelyif you feel a tightness in your chest, have a cough, wheezing, or difficulty breathing immediately after using the medication. These may be symptoms of a condition called bronchospasm (see section 4).
- If your breathing problems worsen or rashes, swelling, or itching appear immediately after using your inhaler, stop taking it and consult your doctor immediately (see section 4).
- If you experience any side effects related to the heart (increased pulse, increased blood pressure, and/or increased symptoms such as chest pain), consult your doctor immediately (see section 4).
- If you experience muscle spasms, muscle weakness, or an abnormal heart rhythm, consult your doctor, as these symptoms may be related to low potassium levels in the blood (see section 4).
Striverdi Respimat is indicated for the maintenance treatment of your chronic obstructive pulmonary disease. It should not be taken to treat a sudden episode of shortness of breath or wheezing.
Do not take Striverdi Respimat with certain medications that contain long-acting beta-adrenergic agonists, such as salmeterol or formoterol.
If you regularly take certain medications called short-acting beta-adrenergic agents, such as salbutamol, continue taking them only to relieve acute symptoms such as shortness of breath.
Children and adolescents
Striverdi Respimat should not be administeredto children or adolescents (under 18 years of age).
Other medications and Striverdi Respimat
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
In particular, inform your doctor or pharmacist if you are taking:
- certain medications for breathing difficulties that are similar to Striverdi Respimat (beta-adrenergic agents). You may be more likely to experience side effects.
- medications called beta-blockers that are used for high blood pressure or heart problems (such as propranolol) or for a problem in the eye known as glaucoma (such as timolol). This may reduce the effect of Striverdi Respimat.
- medications that decrease potassium levels in the blood. For example:
- corticosteroids (e.g., prednisolone),
- diuretics (medications to urinate),
- medications for respiratory problems such as theophylline.
Taking these medications with Striverdi Respimat may cause muscle spasms, muscle weakness, or an abnormal heart rhythm.
- medications called tricyclic antidepressants or MAO inhibitors (such as selegiline or moclobemide), which are used to treat neurological or psychiatric disorders such as Parkinson's disease or depression; using these medications will increase the risk of experiencing heart-related side effects.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before taking this medication.
Driving and using machines
No studies have been conducted on the effects on driving and using machines.
If you feel dizzy while taking Striverdi Respimat, do not drive or use tools or machines.
Striverdi Respimat contains Benzalkonium chloride
This medication contains 0.0011 mg of benzalkonium chloride per puff.
Benzalkonium chloride may cause wheezing and breathing difficulties (bronchospasm), especially in patients with asthma.
3. How to take Striverdi Respimat
Follow the instructions for administration of this medication indicated by your doctor. If you are unsure, consult your doctor or pharmacist again.
Striverdi Respimat should only be used by inhalation.
Dosage
The recommended dose is:
Striverdi Respimat has a 24-hour effect, so you should take Striverdi Respimat only ONCE A DAY, if possible, always at the same time. Each time you use it, inhale TWO PUFFS.
Since COPD is a long-term disease, you should take Striverdi Respimat every day and not just when you have difficulty breathing. Do not take more than the recommended dose.
Make sure you know how to use your Respimat inhaler correctly. The instructions for using the Respimat inhaler are at the end of this package leaflet, see "Instructions for using the Respimat inhaler".
Use in children and adolescents
There is no specific recommendation for the use of Striverdi Respimat in the pediatric population (under 18 years of age).
If you take more Striverdi Respimat than you should
You may have a higher risk of experiencing a side effect such as chest pain, high or low blood pressure, rapid or irregular heartbeat or palpitations, dizziness, nervousness, difficulty sleeping, anxiety, headache, tremor, dry mouth, muscle cramps, nausea, fatigue, malaise, low potassium levels in the blood (which can cause muscle spasms, muscle weakness, or an abnormal heart rhythm), high blood sugar levels, or too much acid in the blood (which can cause symptoms such as nausea, vomiting, weakness, muscle cramps, and rapid breathing).
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take Striverdi Respimat
If you have forgotten to inhale a dose, inhale a single dose the next day at the usual time. Do not take a double dose to make up for the forgotten doses.
If you stop taking Striverdi Respimat
Before stopping treatment with Striverdi Respimat, you should talk to your doctor or pharmacist. If you stop taking Striverdi Respimat, the signs and symptoms of COPD may worsen.
If you have any further questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Striverdi Respimat can cause side effects, although not everyone will experience them.
The side effects described below have been experienced by people who have taken this medication and are listed according to their frequency as uncommon or rare.
Uncommon (may affect up to 1 in 100 people):
- Nasopharyngitis (runny nose)
- Dizziness
- Rash
Rare (may affect up to 1 in 1,000 people):
- Arthralgia (joint pain)
- High blood pressure (hypertension)
You may experience side effects that occur with other medications similar to Striverdi Respimat (beta-adrenergic agents) for treating respiratory problems. These may be rapid or irregular heartbeat or palpitations, chest pain, high or low blood pressure, tremor, headache, nervousness, difficulty sleeping, dizziness, dry mouth, nausea, muscle cramps, fatigue, malaise, low potassium levels in the blood (which can cause muscle spasms, muscle weakness, or an abnormal heart rhythm), high blood sugar levels, or too much acid in the blood (which can cause symptoms such as nausea, vomiting, weakness, muscle cramps, and rapid breathing).
After administration of Striverdi Respimat, immediate allergic reactions such as rash, hives, swelling of the mouth and face, or sudden difficulty breathing (angioedema) or other hypersensitivity reactions may occur. If this happens, stop taking Striverdi Respimat and consult your doctor immediately.
Additionally, as with all inhaled medications, some patients may experience tightness in the chest, coughing, wheezing, or difficulty breathing immediately after inhalation (bronchospasm).
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Agency's online platform: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Striverdi Respimat
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the carton and on the label of the cartridge after EXP. The expiration date is the last day of the month indicated.
Do not freeze.
Shelf life:
Replace the cartridge no more than three months after insertion.
Do not use the Respimat inhaler for more than one year.
Recommended use: 6 cartridges per inhaler.
Note: The functioning of the Respimat inhaler has been demonstrated in tests for 540 puffs (corresponding to 9 cartridges).
Medications should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medication in the SIGRE collection point at your usual pharmacy. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Striverdi Respimat
The active ingredient is olodaterol. The delivered dose is 2.5 micrograms of olodaterol (as hydrochloride) per actuation.
The delivered dose is the dose available to the patient after passing through the mouthpiece.
The other components are:
Benzalkonium chloride, disodium edetate, purified water, and anhydrous citric acid.
Appearance of the Product and Container Contents
Striverdi Respimat 2.5 micrograms consists of a cartridge with an inhalation solution and a Respimat inhaler. Before the first use, you must insert the cartridge into the inhaler.
Individual packaging: 1 Respimat inhaler and 1 cartridge that provides 60 actuations (30 doses).
Triple packaging: 1 Respimat inhaler and 3 cartridges that provide 60 actuations (30 doses) each.
Individual replacement packaging: 1 cartridge that provides 60 actuations (30 doses).
Triple replacement packaging: 3 cartridges that provide 60 actuations (30 doses) each.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
The marketing authorization holder for Striverdi Respimat is:
Boehringer Ingelheim International GmbH
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
The manufacturer of Striverdi Respimat is:
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
Boehringer Ingelheim France
100-104 Avenue de France
75013 Paris
France
Local Representative:
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
Spain
This Medication is Authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) with the Following Names:
Austria, Liechtenstein, Belgium, Luxembourg, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Malta, United Kingdom (Northern Ireland), Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden: Striverdi Respimat
Bulgaria: ????????? ????????
Date of the Last Revision of this Prospectus: December 2024
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es.
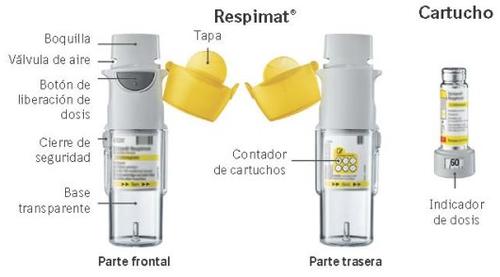
Instructions on How to Use the Respimat Rechargeable Inhaler
Respimat is an inhaler device that generates an aerosol for inhalation. Respimat is for your use only. A cartridge provides several doses. The Respimat rechargeable inhaler allows you to replace the cartridge and can be used with up to 6 cartridges.
Read these instructions before starting to use Striverdi Respimat.
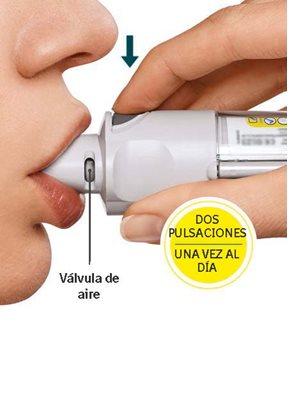
You will need to use this inhaler ONCE A DAY. Each time you use it, perform TWO ACTUATIONS.
- If Striverdi Respimat has not been used for more than 7 days, perform one actuation towards the ground.
- If Striverdi Respimat has not been used for more than 21 days, repeat the steps from 4 to 6 described in “Preparation for Use” until you observe a cloud. Then repeat the steps from 4 to 6 three more times.
How to Maintain Your Respimat Rechargeable Inhaler
Clean the mouthpiece, including the metal part inside it, only with a damp cloth or tissue, at least once a week.
Any slight discoloration of the mouthpiece does not affect the functioning of your Respimat rechargeable inhaler.
If necessary, clean the outside of your Respimat rechargeable inhaler with a damp cloth.
When to Change the Inhaler
When you have used 6 cartridges with the same inhaler, get a new package of Striverdi Respimat containing an inhaler. Do not use the Respimat rechargeable inhaler for more than one year after inserting the first cartridge.

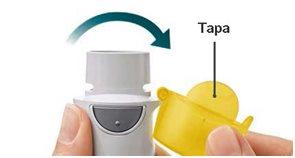
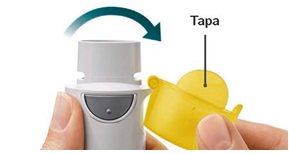
Preparation for Use
|
|
|
|
|
|
|
|
|
|
Your inhaler is now ready to be used and will deliver 60 actuations (30 doses). |
|
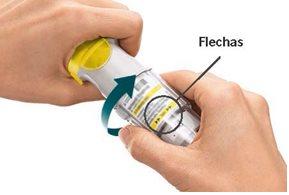
Daily Use
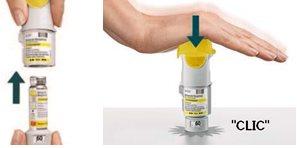
TURN
|
|
OPEN
|
|
ACTUATE
|
|
When to Change the Striverdi Respimat Cartridge
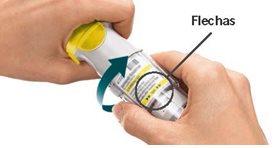
The dose indicator shows how many actuations are left in the cartridge.
 60 actuations remaining.
60 actuations remaining.
 Less than 10 actuations remaining. Get a new cartridge.
Less than 10 actuations remaining. Get a new cartridge.
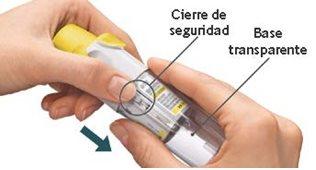
 Your cartridge is empty. Turn the transparent base to loosen it. Your inhaler is now locked. Remove the cartridge from the inhaler. Insert a new cartridge until it clicks (see step 2). The new cartridge will protrude more than the first cartridge (continue with step 3). Remember to replace the transparent base to unlock the inhaler.
Your cartridge is empty. Turn the transparent base to loosen it. Your inhaler is now locked. Remove the cartridge from the inhaler. Insert a new cartridge until it clicks (see step 2). The new cartridge will protrude more than the first cartridge (continue with step 3). Remember to replace the transparent base to unlock the inhaler.
Frequently Asked Questions
It is difficult to insert the cartridge deep enough.
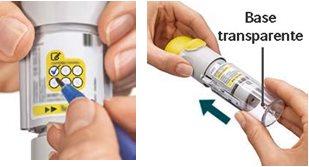
Have you accidentally turned the transparent base before inserting the cartridge?Open the cap, press the dose release button, and then insert the cartridge.
Are you changing the cartridge?The new cartridge will protrude more than the first cartridge. Insert the cartridge until it clicks, then replace the transparent base.
I cannot press the dose release button.
Have you replaced the transparent base?If not, replace the transparent base to unlock the inhaler. The Respimat rechargeable inhaler only works with the transparent base in place.
Have you turned the transparent base?If not, turn the transparent base with a continuous motion until it clicks (half a turn). Does the dose indicator on your cartridge show a white arrow on a red background?Your cartridge is empty. Insert a new cartridge and replace the transparent base.
It is difficult to remove the cartridge once it is empty.
Pull and turn the cartridge at the same time.
I cannot turn or replace the transparent base.
Is the transparent base loose and the dose indicator on your cartridge showing a white arrow on a red background?Your cartridge is empty. Insert a new cartridge.
Have you already turned the transparent base?If you have already turned the transparent base, follow the steps “OPEN” and “ACTUATE” described in “Daily Use” to use your medication.
My Striverdi Respimat has run out too soon.
Have you used Striverdi Respimat as indicated (two actuations/once a day)?Each cartridge will last 30 days if two actuations are performed once a day.
Have you sprayed into the air frequently to check if Striverdi Respimat is working?Once you have prepared Striverdi Respimat for use, it is not necessary to test its functioning by spraying the solution if it is used daily.
Have you removed and replaced the transparent base several times?Do not remove the transparent base before the cartridge is empty. Each time you remove the transparent base without changing the cartridge, the dose counter registers an actuation and the remaining doses are reduced.
My Striverdi Respimat is not spraying.Have you inserted a cartridge?If not, insert a cartridge. Once your Respimat rechargeable inhaler is assembled, do not remove the transparent base or the cartridge until the cartridge is empty.
Have you repeated the steps TURN, OPEN, ACTUATE less than three times after inserting the cartridge?Repeat the steps TURN, OPEN, ACTUATE three times after inserting the cartridge as described in steps 4 to 6 in “Preparation for Use”.
Does the dose indicator on your cartridge show a white arrow on a red background?Your cartridge is empty. Insert a new cartridge.
My Striverdi Respimat rechargeable inhaler is spraying automatically.
Was the cap open when you turned the transparent base?Close the cap, then turn the transparent base.
Did you press the dose release button while turning the transparent base?Close the cap so that the dose release button is covered, then turn the transparent base.
Did you stop turning the transparent base before it clicked?Turn the transparent base with a continuous motion until it clicks (half a turn). The dose counter will count each incomplete turn and the number of remaining doses will be reduced.
Was the cap open when you changed the cartridge?Close the cap, then change the cartridge.
Other Sources of Information
You can access detailed and updated information about how to administer this medication by scanning the QR code included in the section “Instructions on How to Use the Respimat Rechargeable Inhaler” of this prospectus and on the packaging. You can also access this information on the following internet address: https://cima.aemps.es/info/78253

- Country of registration
- Average pharmacy price41.68 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to STRIVERDI RESPIMAT 2.5 micrograms solution for inhalationDosage form: PULMONARY INHALATION, 0.294 mg salmeterol xinafoateActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, salmeterol 50 mcgActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 25 µg salmeterol xinafoate/applicationActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for STRIVERDI RESPIMAT 2.5 micrograms solution for inhalation
Discuss questions about STRIVERDI RESPIMAT 2.5 micrograms solution for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions